What Is Radioactivity And Is It Always Harmful Explained In Really Simple Words

Radioactivity Explained Pdf Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. As its name implies, radioactivity is the act of emitting radiation spontaneously. this is done by an atomic nucleus that, for some reason, is unstable; it "wants" to give up some energy in order to shift to a more stable configuration.

Basics Of Radioactivity Pdf Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. to reach a lower, more stable energy level, it releases energy in the form of radiation. this radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Radioactive decay as previously indicated, large unstable atoms become more stable by emitting radiation to get rid of excess atomic energy (radioactivity). this radiation can be emitted in the form of positively charged alpha particles, negatively charged beta particles, gamma rays, or x rays, as explained below. Radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. the three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. Radioactivity is a very famous term in nuclear physics and chemistry that describes how unstable atoms release certain radiations for the purpose of getting stability.

What Is Radioactivity Pdf Radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. the three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. Radioactivity is a very famous term in nuclear physics and chemistry that describes how unstable atoms release certain radiations for the purpose of getting stability. This process is known as radioactivity and the energy that's released is radiation. after an atom expels energy from the nucleus, the composition of the nucleus changes, and we are left with a different element that is more stable. Radioactivity is a natural phenomenon involving the decay of unstable atomic nuclei, which release energy in the form of radiation. this process helps us understand atomic structures and has applications ranging from medical treatments to energy production. This article will take you through the depths of atomic structure, the mechanics of radioactivity, the different types of decay, the history of its discovery, the technological and medical applications, and the future of this incredible science. This section explores radioactivity and how it relates to a stable atom.

Radioactivity Video Chemistry Ck 12 Foundation This process is known as radioactivity and the energy that's released is radiation. after an atom expels energy from the nucleus, the composition of the nucleus changes, and we are left with a different element that is more stable. Radioactivity is a natural phenomenon involving the decay of unstable atomic nuclei, which release energy in the form of radiation. this process helps us understand atomic structures and has applications ranging from medical treatments to energy production. This article will take you through the depths of atomic structure, the mechanics of radioactivity, the different types of decay, the history of its discovery, the technological and medical applications, and the future of this incredible science. This section explores radioactivity and how it relates to a stable atom.

Solution Radioactivity In Chemistry Fully Explained Studypool This article will take you through the depths of atomic structure, the mechanics of radioactivity, the different types of decay, the history of its discovery, the technological and medical applications, and the future of this incredible science. This section explores radioactivity and how it relates to a stable atom.
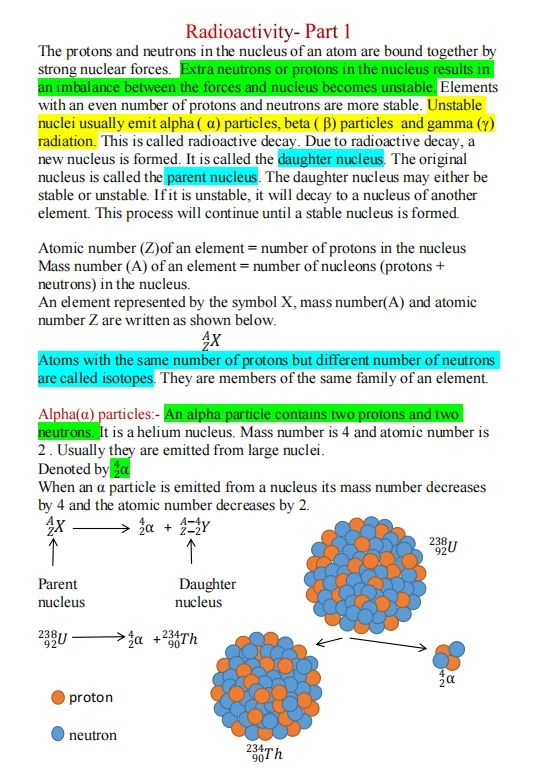
Radioactivity Part 1
Comments are closed.