Testing Containers And Webassembly In Submissions To The Fda R Bloggers
Testing Containers And Webassembly In Submissions To The Fda R Bloggers Now, the r submission working groups, in collaboration with appsilon and posit, are exploring new technologies such as containers and webassembly. in this article, we dive into the details of this exploration. The objective of the r consortium r submission pilot 4 project is to explore the use of novel technologies such as linux containers and webassembly to bundle a shiny application into a self contained package, facilitating a smoother process of both transferring and executing the application.

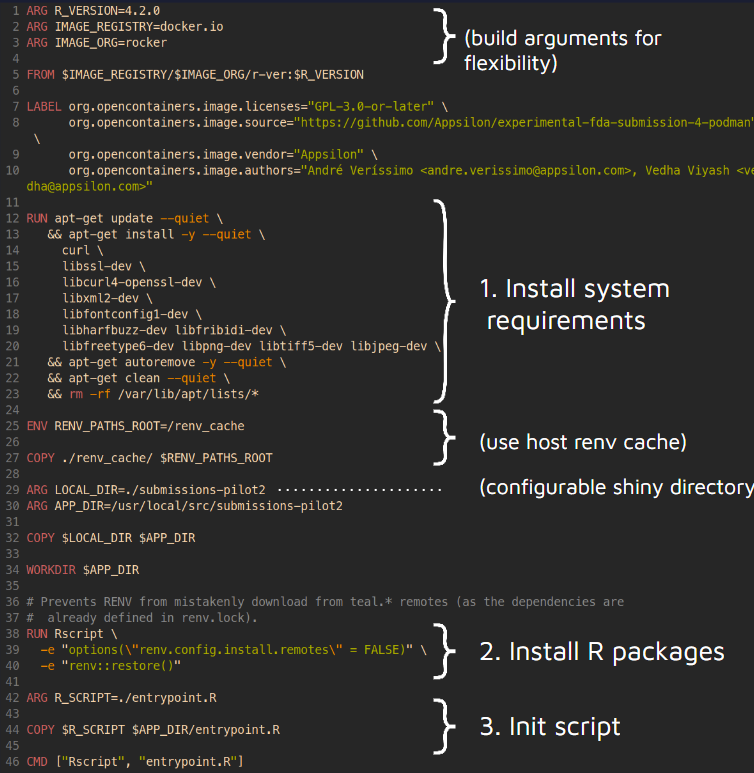
Testing Containers And Webassembly In Submissions To The Fda R Bloggers Curious how containers and webassembly fit into fda submissions? see what pilot 4 revealed in our blog post. this article breaks down the insights shared in a recent shiny gathering featuring ben straub, eric nantz, and sam parmar. Pilot 4 is set to split into two separate submissions, focusing on webassembly and containers, respectively. this approach facilitates a thorough assessment of each technology's capabilities in refining clinical trial submissions. Our main discussion points will be centered around its innovation in executing four pilot submissions to the fda and its exploration of new technologies such as containers and webassembly in collaboration with appsilon and posit. This repository addresses the container technology portion. all submission materials and communications from this pilot are publicly available, with the aim of providing a working example for future r language based fda submissions.
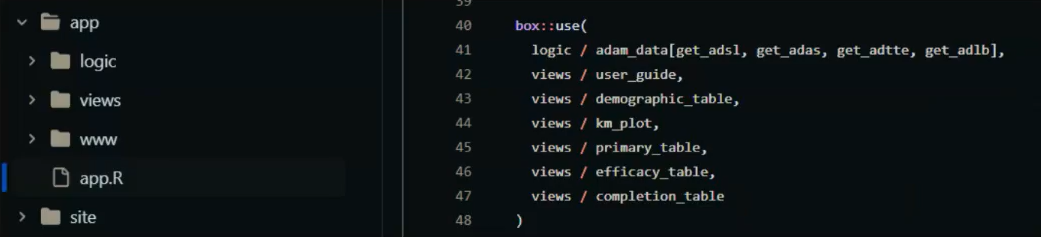
Testing Containers And Webassembly In Submissions To The Fda R Bloggers Our main discussion points will be centered around its innovation in executing four pilot submissions to the fda and its exploration of new technologies such as containers and webassembly in collaboration with appsilon and posit. This repository addresses the container technology portion. all submission materials and communications from this pilot are publicly available, with the aim of providing a working example for future r language based fda submissions. Now, the r submission working groups, in collaboration with appsilon and posit, are exploring new technologies such as containers and webassembly. in this article, we dive into the details of this exploration. Submissions of clinical trial data using shiny and webr are currently being piloted by cross industry statistical programmers and open source software developers in collaboration with the fda. discover how these technologies could expedite therapeutic reviews, and how you can get involved. The r consortium submission working group is excited to announce a new pilot 5 that aims to deliver an r based submission to the fda using dataset json. this post also includes plans for additional launches for 2025 2026, some news on pilot 4 (containers and webassembly) and a few other goodies!. To our knowledge, this is the first publicly available r based fda submission package. we hope this submission package and our learnings can serve as a good reference for future r based regulatory submissions from different sponsors.
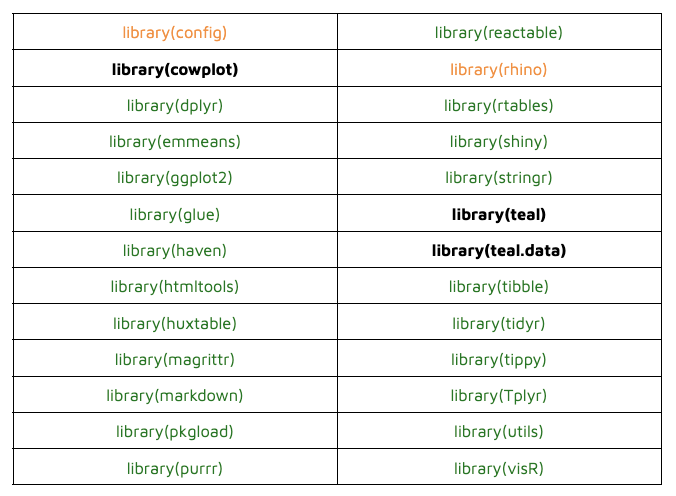
Testing Containers And Webassembly In Submissions To The Fda R Bloggers Now, the r submission working groups, in collaboration with appsilon and posit, are exploring new technologies such as containers and webassembly. in this article, we dive into the details of this exploration. Submissions of clinical trial data using shiny and webr are currently being piloted by cross industry statistical programmers and open source software developers in collaboration with the fda. discover how these technologies could expedite therapeutic reviews, and how you can get involved. The r consortium submission working group is excited to announce a new pilot 5 that aims to deliver an r based submission to the fda using dataset json. this post also includes plans for additional launches for 2025 2026, some news on pilot 4 (containers and webassembly) and a few other goodies!. To our knowledge, this is the first publicly available r based fda submission package. we hope this submission package and our learnings can serve as a good reference for future r based regulatory submissions from different sponsors.

Testing Containers And Webassembly In Submissions To The Fda R Bloggers The r consortium submission working group is excited to announce a new pilot 5 that aims to deliver an r based submission to the fda using dataset json. this post also includes plans for additional launches for 2025 2026, some news on pilot 4 (containers and webassembly) and a few other goodies!. To our knowledge, this is the first publicly available r based fda submission package. we hope this submission package and our learnings can serve as a good reference for future r based regulatory submissions from different sponsors.
Testing Containers And Webassembly In Submissions To The Fda R Bloggers
Comments are closed.