Solved Use This Data To Determine Ahcl Phcl Where The Chegg

Solved Use This Data To Determine Ahcl Phcl Where The Chegg There are 2 steps to solve this one. for the equilibrium reaction hcl (g) ↽ − − ⇀ hcl (aq), we need to determine the ratio of the activity of hcl in the. Question: vi. pre lab exercises use the data below to prepare a piot of am vs c^1 2 for hcl,and determine ahcl. be sure to eliminate values that are not in the linear region conductivity data for hci at 25 degree c vdi = 50.00 ml cstockhcl = 0.50613 m here’s the best way to solve it.
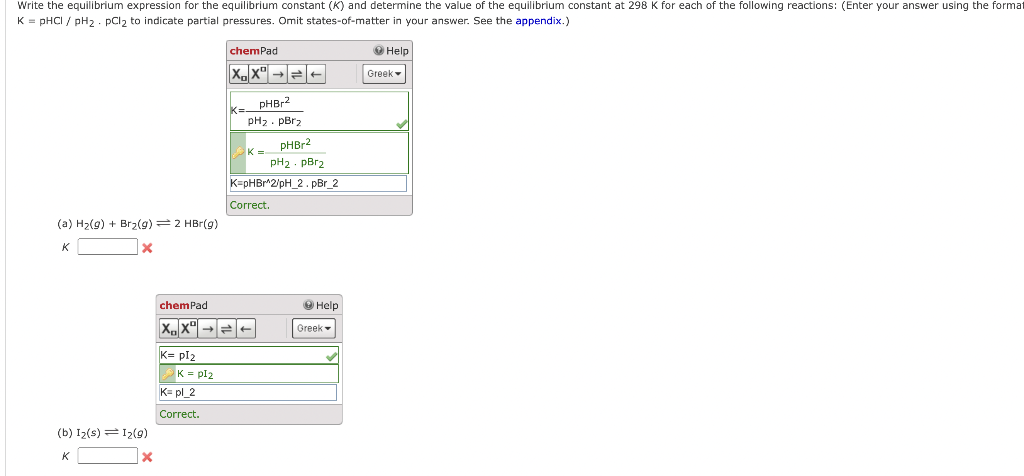
Solved K Phcl Ph2站ûcl2 To Indicate Partial Pressures Omit Chegg Using the sample weights, solution volumes, equilibrium acid concentrations, and original aid concentrations, calculate n, the number of moles of acid adsorbed per gram of adsorbent. Our expert help has broken down your problem into an easy to learn solution you can count on. In this question, you will calculate the expected phs of your solutions, and compare them to your measurements. (a) use the data from part a to fill in the following table. (enter your calculated phs to two decimal places. What ph results from dissolving an oxide in water? see separate problem list. see separate video list.

Solved Part A Use The Information Provided To Determine Ahxn Chegg In this question, you will calculate the expected phs of your solutions, and compare them to your measurements. (a) use the data from part a to fill in the following table. (enter your calculated phs to two decimal places. What ph results from dissolving an oxide in water? see separate problem list. see separate video list. • compare theoretical ph and experimental ph from the given data, where a is the concentration. ahcl = ah acl ph = log [h*) = log [a] theoretical concentration 0.9 m hci 2.5 m hci experimental 0.05 out of range. Section 18.4. problems involving weak acid equilibria two types: (a) given conc’s, find ka ; (b) given ka, find conc’s. method: write balanced equation and set up reaction table let x be the unknown conc. solve. use quadratic equation if necessary. Question: consider the following reaction and data: 4 hcl (g) sif4 (g) sicl4 (1) 4 hf (g) ag° = 232.6 kj what is ag at 298 k when phcl = 5.50 atm, psif4 = 2.25 atm, and phf = 0.0725 atm?. 2. ^ chegg survey fielded between april 23 april 25, 2021 among customers who used chegg study and chegg study pack in q1 2020 and q2 2021. respondent base (n=745) among approximately 144,000 invites.

Help Chegg • compare theoretical ph and experimental ph from the given data, where a is the concentration. ahcl = ah acl ph = log [h*) = log [a] theoretical concentration 0.9 m hci 2.5 m hci experimental 0.05 out of range. Section 18.4. problems involving weak acid equilibria two types: (a) given conc’s, find ka ; (b) given ka, find conc’s. method: write balanced equation and set up reaction table let x be the unknown conc. solve. use quadratic equation if necessary. Question: consider the following reaction and data: 4 hcl (g) sif4 (g) sicl4 (1) 4 hf (g) ag° = 232.6 kj what is ag at 298 k when phcl = 5.50 atm, psif4 = 2.25 atm, and phf = 0.0725 atm?. 2. ^ chegg survey fielded between april 23 april 25, 2021 among customers who used chegg study and chegg study pack in q1 2020 and q2 2021. respondent base (n=745) among approximately 144,000 invites.
Comments are closed.