Solved The Gas Ammonia A Is Diffusing At Steady State Through N2 B

Solved Ammonia Gas Is Diffusing Through N2 Under Steady State The gas ammonia (a) is diffusing at steady state through n2 (b) by equimolar counterdiffusion in a conduit 1.22 m long at 25°c and a total pressure of 101.32 kpa abs. To calculate the flux of ammonia (nh₃) through nitrogen (n₂), we will apply fick's first law of diffusion. this law states that the diffusion flux, or the amount of substance flowing through a surface per unit area per unit time, is proportional to the concentration gradient.
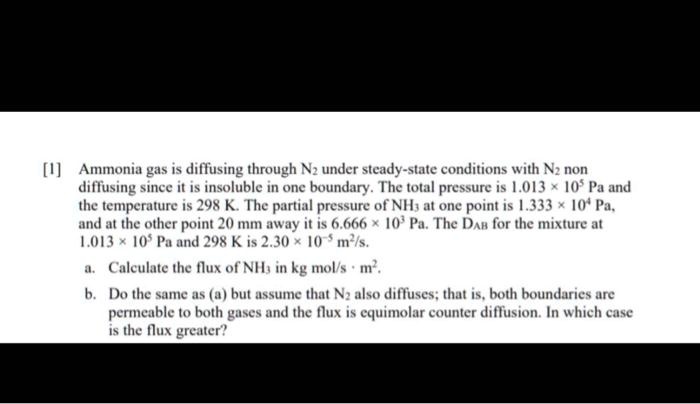
Solved Ammonia Gas Diffuses Through N2 Under Steady State Conditions The gas ammonia is diffusing at steady state through nitrogen by equimolar counterdiffusion in a conduit 1 m long at 25 °c and a total pressure of 101 kpa. the partial pressure of ammonia is 25 kpa at the left end and 5 kpa at the other end. Fick's law of diffusion: this law describes the diffusion of a species through a mixture due to a concentration gradient. we'll use different forms of fick's law depending on whether component b (n2) is diffusing or non diffusing. El amoníaco gaseoso (a) se difunde en estado estacionario a través de n2 (b) por contradifusión equimolar en un conducto de 1,22 m de largo a 25 ° c y a una presión total de 101,32 kpa absoluta. Ammonia (a) is diffusing at steady state through n 2 (b) by equimolar counter diffusion in a conduit 1.22 m long at 25°c and a total pressure of 101.32 kpa abs. the partial pressure of ammonia at the left end is 25.33 kpa and at the other end 5.066 kpa.

Solved Ammonia Gas Is Diffusing Through Nitrogen Under Chegg El amoníaco gaseoso (a) se difunde en estado estacionario a través de n2 (b) por contradifusión equimolar en un conducto de 1,22 m de largo a 25 ° c y a una presión total de 101,32 kpa absoluta. Ammonia (a) is diffusing at steady state through n 2 (b) by equimolar counter diffusion in a conduit 1.22 m long at 25°c and a total pressure of 101.32 kpa abs. the partial pressure of ammonia at the left end is 25.33 kpa and at the other end 5.066 kpa. Diffusion in a nonuniform cross sectional area. the gas ammonia (a) is diffusing at steady state through n 2 (b) by equimolar counterdiffusion in a conduit 1.22 m long at 25°c and a total pressure of 101.32 kpa abs. The gas ammonia (a) is diffusing at steady state through nitrogen (b) by equimolar counterdiffusion in a conduit 1.22 m long at 25 °c and a total pressure of 101.32 kpa. Calculate the rate of diffusion of ammonia through a film of gas 0.5 mm thick, when ammonia concentration changes across the film from 12% to 7%. the diffusivities at 200 °c and 1 atm pressure are dab = 5.391 x 10^9 m^2 s and dec = 1.737 x 10^4 m^2 s, respectively. Ensure that concentrations are converted correctly using the ideal gas law. common errors include miscalculating distances or pressures, which can significantly affect the resulting flux.
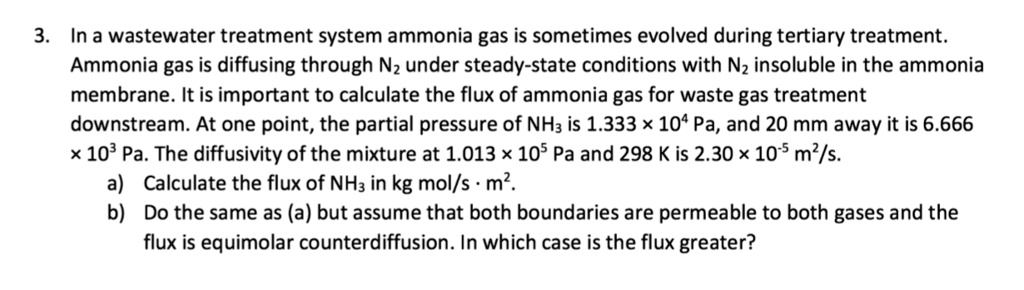
Solved In A Wastewater Treatment System Ammonia Gas Is Sometimes Diffusion in a nonuniform cross sectional area. the gas ammonia (a) is diffusing at steady state through n 2 (b) by equimolar counterdiffusion in a conduit 1.22 m long at 25°c and a total pressure of 101.32 kpa abs. The gas ammonia (a) is diffusing at steady state through nitrogen (b) by equimolar counterdiffusion in a conduit 1.22 m long at 25 °c and a total pressure of 101.32 kpa. Calculate the rate of diffusion of ammonia through a film of gas 0.5 mm thick, when ammonia concentration changes across the film from 12% to 7%. the diffusivities at 200 °c and 1 atm pressure are dab = 5.391 x 10^9 m^2 s and dec = 1.737 x 10^4 m^2 s, respectively. Ensure that concentrations are converted correctly using the ideal gas law. common errors include miscalculating distances or pressures, which can significantly affect the resulting flux.
Comments are closed.