Solved Part A Latent Heat Of Fusionpart Banalysis Part A Chegg

Solved Part A Latent Heat Of Fusionpart Banalysis Part A Chegg Part a latent heat of fusion: data was recorded using the above shown apparatus setup for measuring the latent heat of fusion for water. using a temperature sensor, the data collection program displayed the graph of temperature versus time, as shown above. The measured values of the various masses and temperatures are given in the below table. 2. calculate the experimental latent heat of fusion for water (lf) using the equation in the theory section and record the result in the below table. 3. compare the experimental value to the accepted value given in the below tables by.
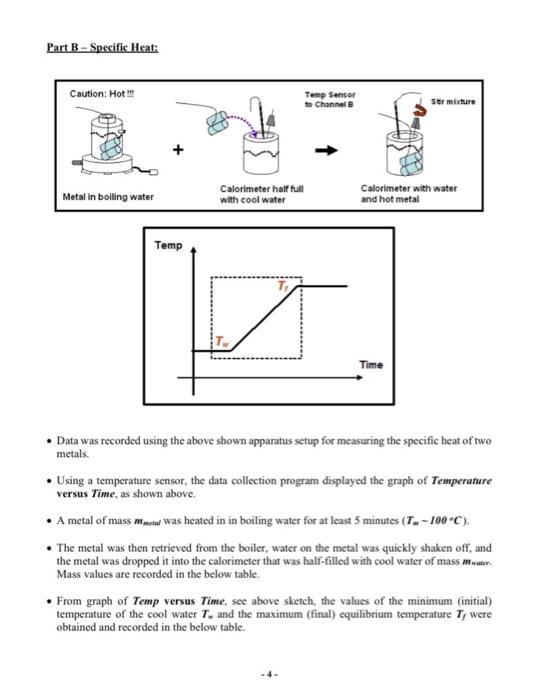
Solved Part A Latent Heat Of Fusionpart Banalysis Part A Chegg Object: to determine the latent heat of fusion of ice and the latent heat of vaporization of steam; to do a full analysis of random and other errors which may affect the experiment; to discuss other possible systematic errors. To determine the latent heat of fusion of water (lf), we place ice in a calorimeter that contains warm water. the ice starts at approximately 0∘c, melts into water, and then warms up to the same final temperature as the warm water. Consider the following temperature time graph when a solid is heated. which part of the graph involves latent heat of fusion? temperature 100 a. part 1 b. part 11 8 pts c. part iii 100 000 d. part iv i iii iv time (s) how much heat is required to raise the temperature of a 200 g glass ball from 15.0°c to show transcribed image text. Calculate the experimental specific heat of the two metals ( cmetal ) using the equation in the theory section and record the results in the below table.

Solved Part A Latent Heat Of Fusionpart Banalysis Part A Chegg Consider the following temperature time graph when a solid is heated. which part of the graph involves latent heat of fusion? temperature 100 a. part 1 b. part 11 8 pts c. part iii 100 000 d. part iv i iii iv time (s) how much heat is required to raise the temperature of a 200 g glass ball from 15.0°c to show transcribed image text. Calculate the experimental specific heat of the two metals ( cmetal ) using the equation in the theory section and record the results in the below table. Search our library of 100m curated solutions that break down your toughest questions. ask one of our real, verified subject matter experts for extra support on complex concepts. test your knowledge anytime with practice questions. create flashcards from your questions to quiz yourself. In this part of the experiment you will measure the latent heat of fusion of water. this is the heat required to change the state of water from a solid (ice) to a liquid. The heat required to change 1.0 kg of a solid to a liquid (or vice versa) is the latent heat of fusion. solve problems about this concept. To calculate the latent heat of fusion, start by setting up an equation based on the principle of conservation of energy: the heat gained by ice equals the heat lost by the cup and the water.
Comments are closed.