Solved Measurement Deducing The Unit Missing From The Chegg
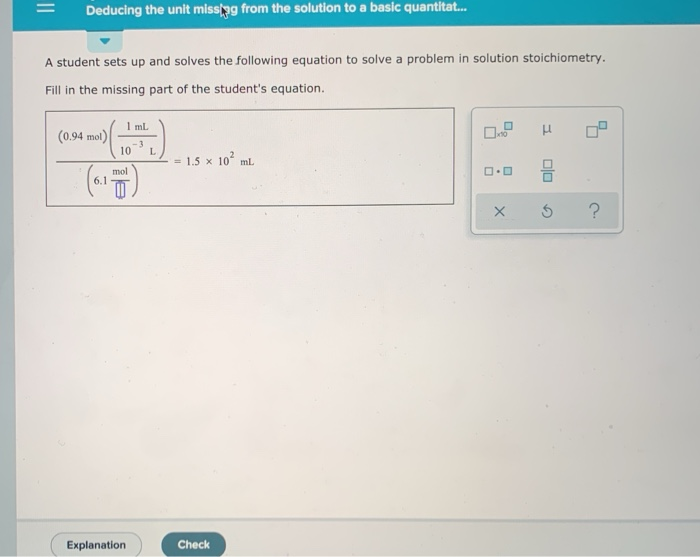
Solved Deducing The Unit Missing From The Solution To A Chegg Deducing the unit missing from the solution to a basic a student sets up and solves the following equation to solve a problem in solution stoichiometry. fill in the missing part of the student's equation. To determine the missing unit in the equation presented in your question, we first need to analyze the components of the equation based on stoichiometry and unit conversion.
Solved Deducing The Unit Missing From The Solution To A Chegg Deducing the unit missing from the solution to a basic quantitative problem a student sets up and solves the following equation to solve a problem in solution stoichiometry. From the equation, we can see that the missing unit is the unit of the quantity being multiplied by 6. to find the missing unit, we need to divide both sides of the equation by 6:. A student sets up the following equation to convert a measurement (the ? stands for a number the student is going to calculate:) fill in the missing part of this equation. Skip the cable setup & start watching tv today for free. then save $23 month for 2 mos.

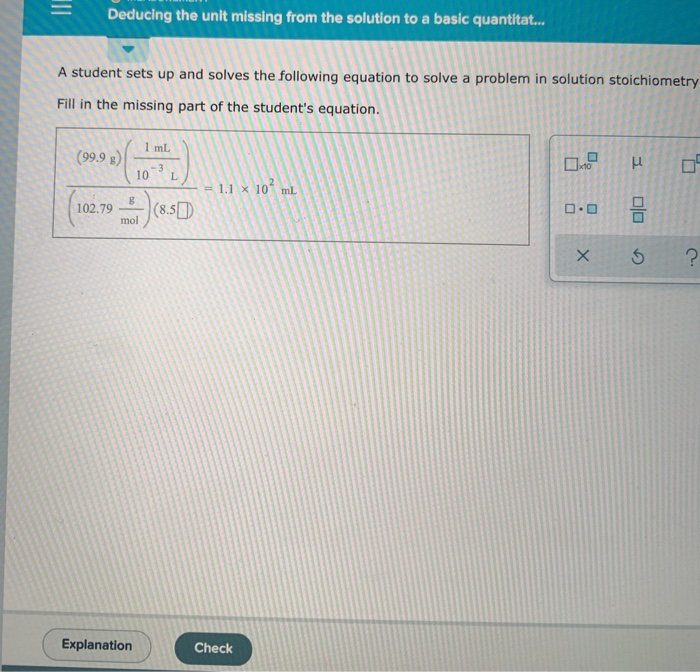
Solved O Measurement Deducing The Unit Missing From The Chegg A student sets up the following equation to convert a measurement (the ? stands for a number the student is going to calculate:) fill in the missing part of this equation. Skip the cable setup & start watching tv today for free. then save $23 month for 2 mos. Measurement: deducing the unit missing from the solution to a basic quantitative problem, the student sets up and solves the following equation to solve a problem in solution stoichiometry. Question: measurement and matter deducing the unit missing from the solution to a basic a student sets up and solves the following equation to solve a problem in solution stoichiometry. Suppose a later and more reliable measurement gives 0.770s for the period of the same star. decide which of the earlier measurements was the most accurate, and which was the most precise. The missing unit in the student's equation is mol−1. this unit allows the quantity in the brackets to convert correctly into grams, matching the target result of 53 g.
Comments are closed.