Solved How Do I Calculate The Concentration For The Unknown Chegg
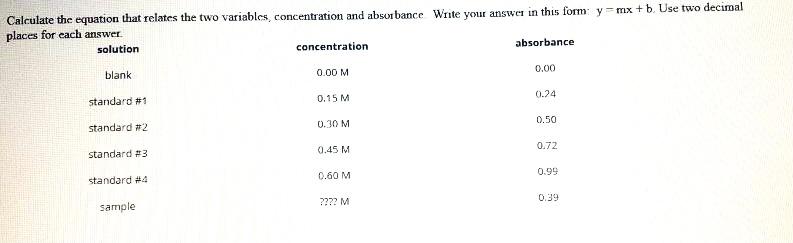
Solved Calculate The Unknown Concentration And Calculate The Chegg Here’s the best way to solve it. the formula for calculating the unknown value would be: were, a = absorbance; epsilon = molar absorption coefficient; l = beam path lengt …. You’re looking for the equivalence point on your titration. this is where the ph changes a lot with just a small addition of titrant. at this point you’ve added in as many moles of strong base as you had weak acid. use the concentration of the strong base and its volume added to find the moles.

Solved Calculate The Concentration Of The Unknown Naoh Then Chegg Concentration is determined mathematically by taking the mass, moles, or volume of solute and dividing it by the mass, moles, or volume of the solution (or less commonly, the solvent). To calculate the concentration of an unknown solution, you typically use a process called titration. here's a general step by step guide: first, you need to prepare a solution of known concentration, called the titrant. then, you measure a specific volume of the unknown solution into a flask. Data collected during a titration allows chemists to determine a solution’s unknown concentration. concordant titres should be used when calculating a mean volume used to neutralise a solution. The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line.

Solved Calculate The Concentration Of An Unknown Solution In Chegg Data collected during a titration allows chemists to determine a solution’s unknown concentration. concordant titres should be used when calculating a mean volume used to neutralise a solution. The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line. There are two basic ways of reporting the concentration of a solute in a solvent, by reporting the mass of solute in a given volume, or the number of moles of solute in a given volume. Concentration is a fundamental concept in chemistry that refers to the amount of a substance (solute) present in a given volume of solution. it is typically expressed in terms of molarity (moles of solute per liter of solution) or percentage concentration (mass of solute per total mass of solution). We also have a tool to help you calculate the concentration of an unknown sample with the calibration curve! what is mass percentage concentration formula? mass percent concentration (wt%) is one of the types of percentage concentration. it is defined as the ratio of the solute's mass to the solution's total mass. Question: how do i find the concentration of the unknown? how do i find the concentration of the unknown? there are 3 steps to solve this one.

Solved How Do I Calculate The Concentration For The Unknown Chegg There are two basic ways of reporting the concentration of a solute in a solvent, by reporting the mass of solute in a given volume, or the number of moles of solute in a given volume. Concentration is a fundamental concept in chemistry that refers to the amount of a substance (solute) present in a given volume of solution. it is typically expressed in terms of molarity (moles of solute per liter of solution) or percentage concentration (mass of solute per total mass of solution). We also have a tool to help you calculate the concentration of an unknown sample with the calibration curve! what is mass percentage concentration formula? mass percent concentration (wt%) is one of the types of percentage concentration. it is defined as the ratio of the solute's mass to the solution's total mass. Question: how do i find the concentration of the unknown? how do i find the concentration of the unknown? there are 3 steps to solve this one.
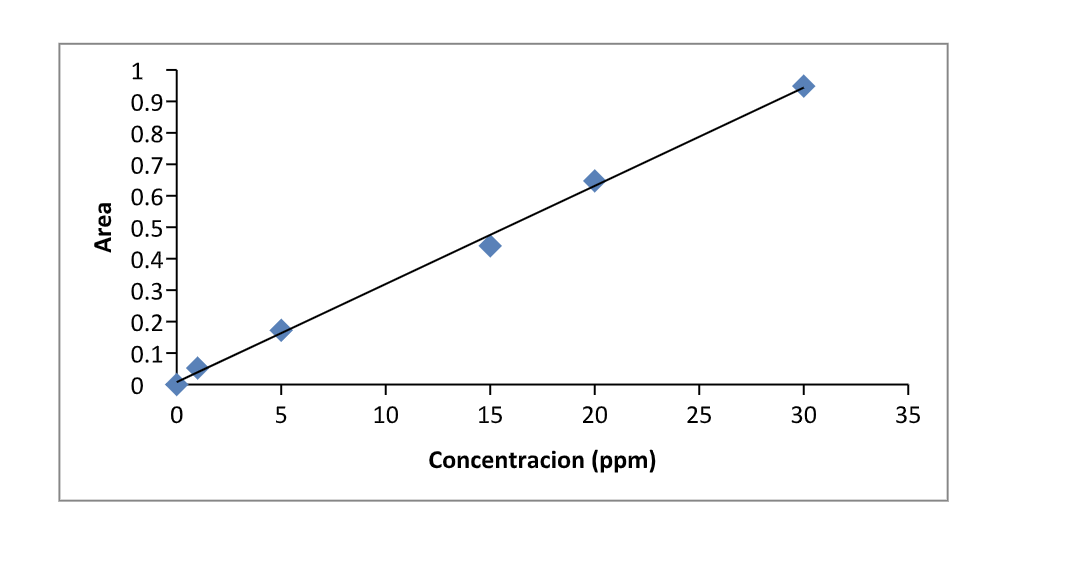
Solved Calculate The Concentration Of An Unknown Sample Chegg We also have a tool to help you calculate the concentration of an unknown sample with the calibration curve! what is mass percentage concentration formula? mass percent concentration (wt%) is one of the types of percentage concentration. it is defined as the ratio of the solute's mass to the solution's total mass. Question: how do i find the concentration of the unknown? how do i find the concentration of the unknown? there are 3 steps to solve this one.
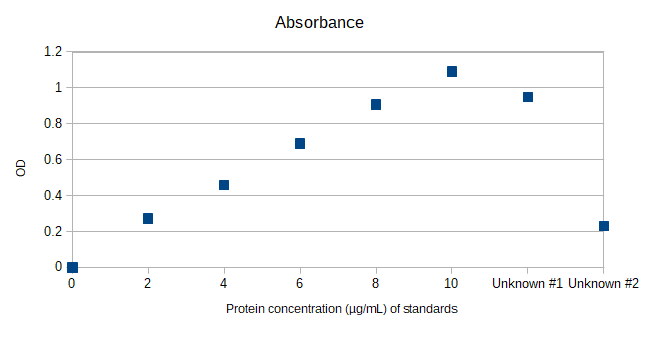
Solved Calculate The Concentration Of Unknown 1 And Unknown Chegg
Comments are closed.