Solved Empirical Forms Ionic Formula Of Ionic Compound Compound
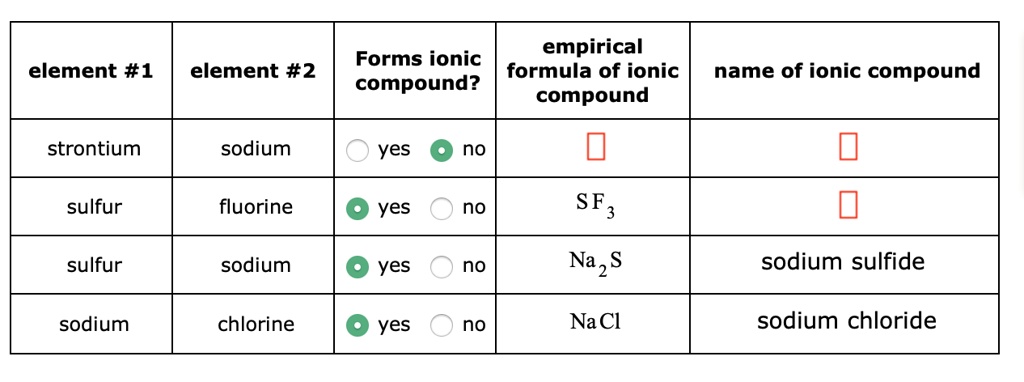
Solved Empirical Forms Ionic Formula Of Ionic Compound Compound With our user friendly interface and advanced algorithms, you can quickly and easily calculate the empirical formula of any ionic compound, whether you're a student, researcher, or professional. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. an ionic formula, like \ (\ce {nacl}\), is an empirical formula. this formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions.

Solved Empirical Forms Ionic Formula Of Ionic Compound Compound Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. fluorine is non metal and sulphur is non metal. not the question you’re looking for? post any question and get expert help quickly. Understand what empirical formula is for your igcse chemistry exam. use worked examples to determine the empirical formula for various compounds. learn more. The arrangement of the periodic table by increasing atomic number places elements with similar chemical properties in the same columns (groups). elements in the same group have the same number of valence electrons and typically tend to form similarly charged ions. metallic cations bond with nonmetallic anions to form ionic compounds. the empirical formula gives the ratio of cations to anions. Atomic chlorine, fluorine, sodium (element #1), sulfur, sodium (element #2), potassium, and barium are all elements. yes, they can form ionic compounds. the formula for an ionic compound is empirical and has an ionic name. let's fill in the table here, but we'll start with the definition of an ionic compound ionic compound.

Solved Forms Ionic Compound Empirical Formula Of Ionic Compound The arrangement of the periodic table by increasing atomic number places elements with similar chemical properties in the same columns (groups). elements in the same group have the same number of valence electrons and typically tend to form similarly charged ions. metallic cations bond with nonmetallic anions to form ionic compounds. the empirical formula gives the ratio of cations to anions. Atomic chlorine, fluorine, sodium (element #1), sulfur, sodium (element #2), potassium, and barium are all elements. yes, they can form ionic compounds. the formula for an ionic compound is empirical and has an ionic name. let's fill in the table here, but we'll start with the definition of an ionic compound ionic compound. Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table. cr3 no3 nh4 oh na no3 sure, i can help with that. the name and empirical formula of an ionic compound is determined by the ions that make up the compound. Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula. instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. When the subscripts in a chemical formula represent the simplest ratio of the kinds of atoms in the compound, the formula is called an empirical formula. most ionic compounds are described with empirical formulas. In a procedure called elemental analysis, an unknown compound can be analyzed in the laboratory in order to determine the percentages of each element contained within it.
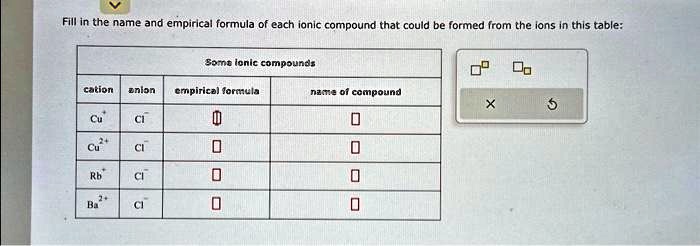
Solved Texts What Is The Empirical Formula And Compound Name Of Each Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table. cr3 no3 nh4 oh na no3 sure, i can help with that. the name and empirical formula of an ionic compound is determined by the ions that make up the compound. Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula. instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. When the subscripts in a chemical formula represent the simplest ratio of the kinds of atoms in the compound, the formula is called an empirical formula. most ionic compounds are described with empirical formulas. In a procedure called elemental analysis, an unknown compound can be analyzed in the laboratory in order to determine the percentages of each element contained within it.
Comments are closed.