Solved Determine The Concentration Ug Ml Of The Unknown Chegg
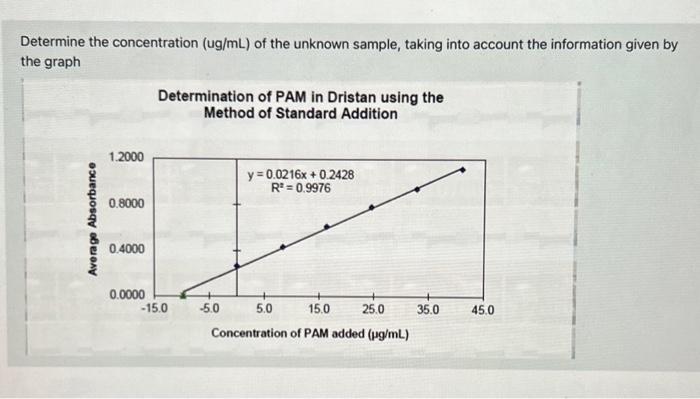
Determine The Concentration Ug Ml Of The Unknown Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. You then work back through your dilutions to get, in this case, ug ml of analyte in the original sample preparation flask. next you multiply by ml of that flask to get ug of analyte.

Solved Determine The Concentration Ug Ml Of The Unknown Chegg Calibration graph is constructed by plotting absorbance at a given wavelength versus concentration for a series of standard solutions whose concentrations are accurately known. How to determine a concentration of unknown protein sample? determining the concentration of an unknown protein sample is a common task in biochemistry and molecular biology. there are several methods to do this, but one of the most common is the bradford protein assay. To determine the concentration of the unknown sample, we can use the beer lambert law. the law states that the absorbance of a solution is equal to the product of the molar absorptivity, the path length, and the concentration of the solute. The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line.

Solved Determine The Concentration Ug Ml Of The Unknown Chegg To determine the concentration of the unknown sample, we can use the beer lambert law. the law states that the absorbance of a solution is equal to the product of the molar absorptivity, the path length, and the concentration of the solute. The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line. Study with quizlet and memorize flashcards containing terms like how do you determine the protein amount (ug), how do you determine the concentration of the diluted sample (ug ul)?, how do you calculate the concentration of the unknown? (ug ul) and more. Free practice questions for high school chemistry identifying unknown concentration. includes full solutions and score reporting. Our expert help has broken down your problem into an easy to learn solution you can count on. question: hplc was used to determine the concentration (ug l) of an unknown sample. Determine the molar mass of an unknown monoprotic acid to two decimal places if 15.4 ml of a 0.098 m naoh solution were used to titrate 0.212 g of the unknown acid.
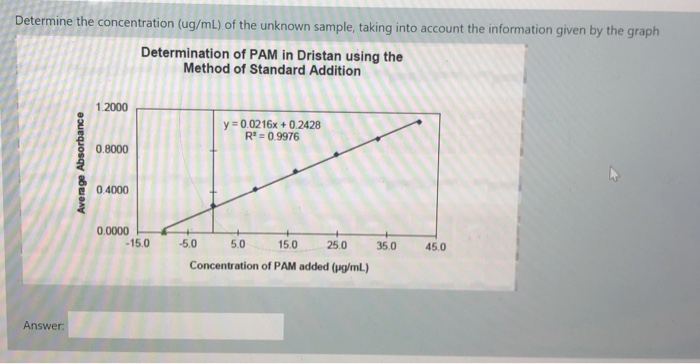
Solved Determine The Concentration Ug Ml Of The Unknown Chegg Study with quizlet and memorize flashcards containing terms like how do you determine the protein amount (ug), how do you determine the concentration of the diluted sample (ug ul)?, how do you calculate the concentration of the unknown? (ug ul) and more. Free practice questions for high school chemistry identifying unknown concentration. includes full solutions and score reporting. Our expert help has broken down your problem into an easy to learn solution you can count on. question: hplc was used to determine the concentration (ug l) of an unknown sample. Determine the molar mass of an unknown monoprotic acid to two decimal places if 15.4 ml of a 0.098 m naoh solution were used to titrate 0.212 g of the unknown acid.
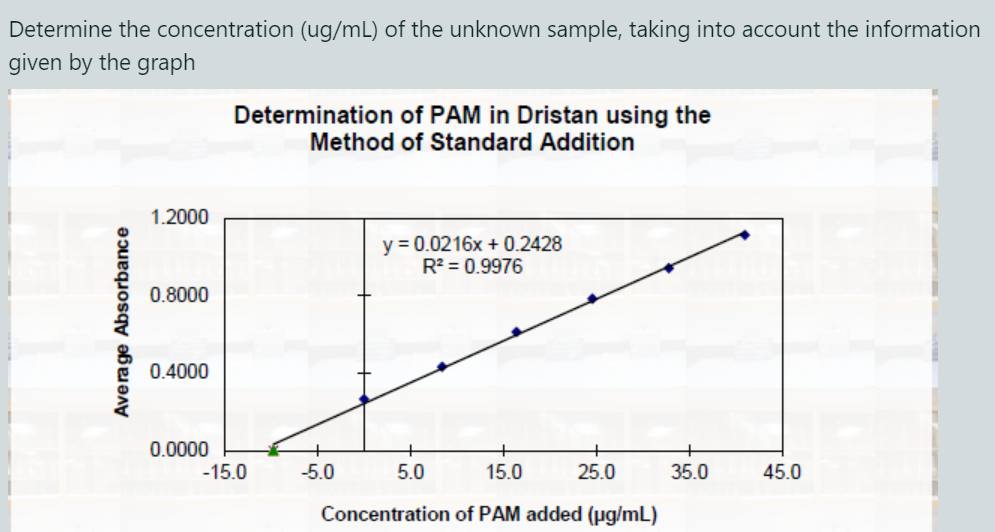
Determine The Concentration Ug Ml Of The Unknown Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. question: hplc was used to determine the concentration (ug l) of an unknown sample. Determine the molar mass of an unknown monoprotic acid to two decimal places if 15.4 ml of a 0.098 m naoh solution were used to titrate 0.212 g of the unknown acid.
Comments are closed.