Solved Determine The Concentration Of An Unknown Solution Chegg
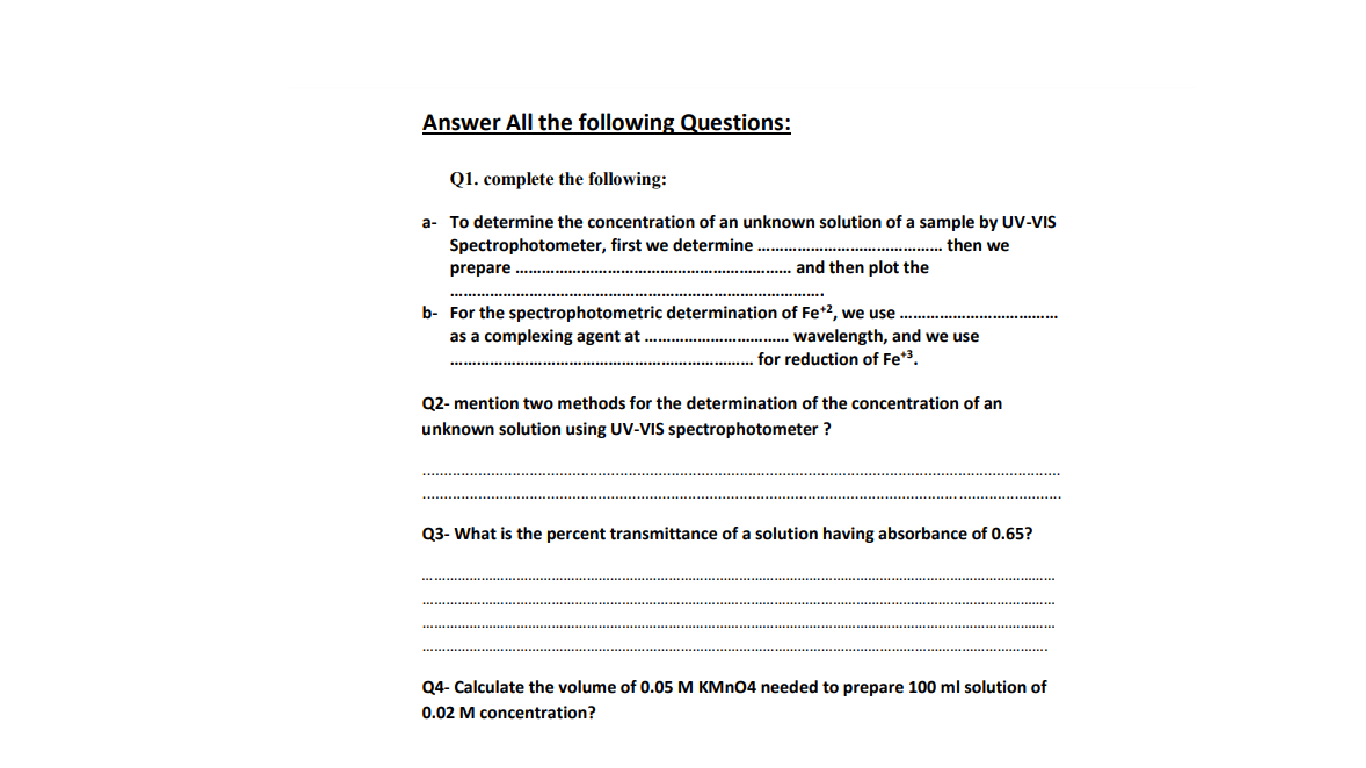
Solved A To Determine The Concentration Of An Unknown Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. calculate the concentration of an unknown solution in a concentration cell. there are 3 steps to solve this one. the given concentration cell is made by hydrogen electrodes. the cathode is a standard hydrogen elect not the question you’re looking for?. Learning objectives calculate percentage concentration (m m, v v, m v), ppm and ppb. calculate the molarity of a solution. use concentration units to calculate the amount of solute in a solution. use molarity to determine quantities in chemical reactions. determine the resulting concentration of a diluted solution.
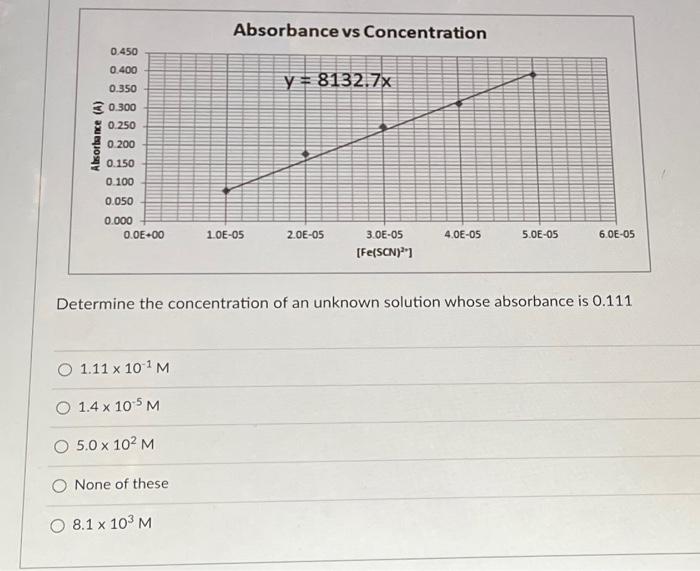
Solved Determine The Concentration Of An Unknown Solution Chegg Refractometry 7.1 to determine the concentration of an unknown solution of ethanol 7 standard mixtures of ethanol and water was prepared. the refractive index of these standards, water, pure ethanol and the unknown was determined. To calculate the concentration of an unknown solution, you typically use a process called titration. here's a general step by step guide: first, you need to prepare a solution of known concentration, called the titrant. then, you measure a specific volume of the unknown solution into a flask. The task is to determine the concentration of an unknown solution. this is accomplished by comparing the intensity of the color of the unknown solution to a series of known solutions. start by calculating the recipes for making the known solutions on pages 2 3. Use the concentration of the strong base and its volume added to find the moles. if your acid has one acidic hydrogen (monoprotic, if your teacher didn’t say then it’s probably monoprotic) then the moles of acid equals the moles of base.

Solved In Order To Determine The Concentration Of An Unknown Chegg The task is to determine the concentration of an unknown solution. this is accomplished by comparing the intensity of the color of the unknown solution to a series of known solutions. start by calculating the recipes for making the known solutions on pages 2 3. Use the concentration of the strong base and its volume added to find the moles. if your acid has one acidic hydrogen (monoprotic, if your teacher didn’t say then it’s probably monoprotic) then the moles of acid equals the moles of base. The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line. Free practice questions for high school chemistry identifying unknown concentration. includes full solutions and score reporting. Use the ti graph link cable and program to transfer the graph of absorbance vs. concentration (including the interpolated unknown concentration) to a laptop computer. Please calculate the calibration curve and find the approximate concentration of the unknown solution based on the absorbance concentration values of the five solution samples.
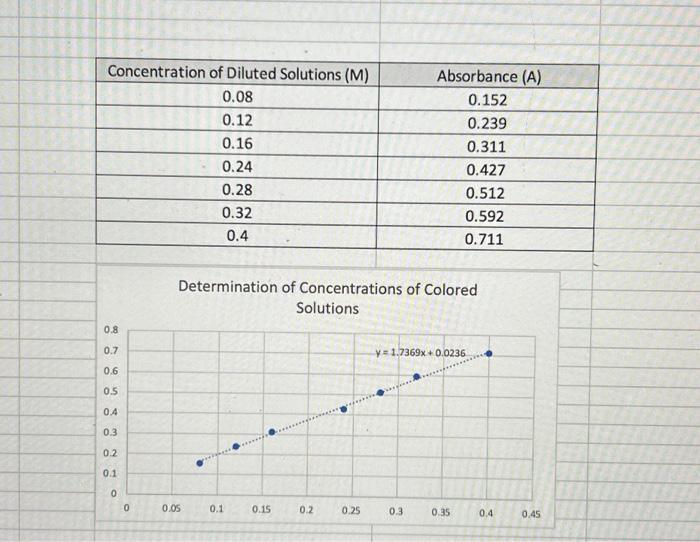
Solved Solve 2 Determine The Concentration Of The Unknown Chegg The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line. Free practice questions for high school chemistry identifying unknown concentration. includes full solutions and score reporting. Use the ti graph link cable and program to transfer the graph of absorbance vs. concentration (including the interpolated unknown concentration) to a laptop computer. Please calculate the calibration curve and find the approximate concentration of the unknown solution based on the absorbance concentration values of the five solution samples.
Comments are closed.