Solved Decomposition Reaction Lab Report Date Experiment Chegg

Solved Decomposition Reaction Lab Report Date Experiment Chegg Question: decomposition reaction lab report date experiment performed: need the yields, results, and conclusion answered show transcribed image text. Sodium bicarbonate ( nahco 3 ), or baking soda is a chemical that can undergo a decomposition reaction when heated. there are three theoretically possible chemical reactions that could occur during the thermal decomposition of baking soda.
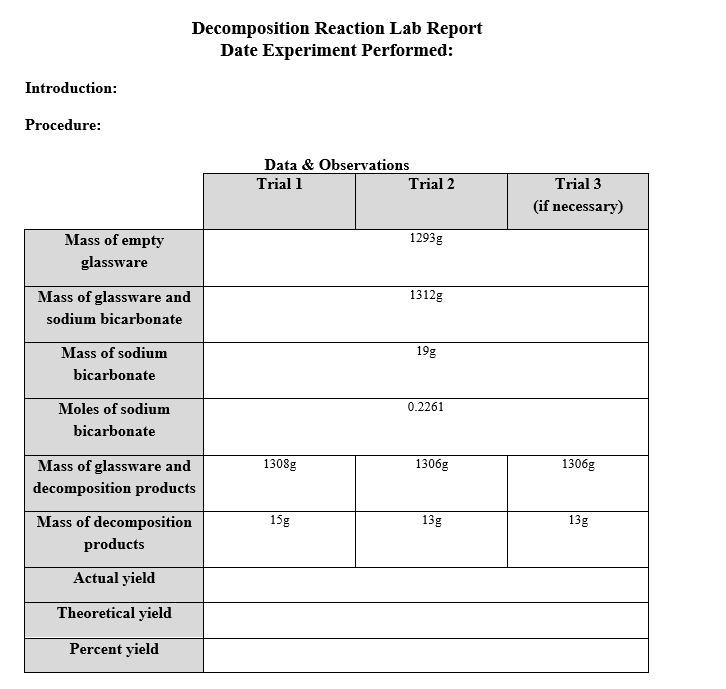
Solved Decomposition Reaction Lab Report Date Experiment Chegg In the given experiment, the results included the masses of empty glassware, glassware with sodium bicarbonate, glassware with decomposition products, and the mass of sodium bicarbonate used in each trial. Our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. Experiment 14 lab report on decomposition reaction rates course: general chemistry lab ii (che113) 408 documents university: university of kentucky. This demo makes use of the catalytic decomposition of hydrogen peroxide to produce a column of steam out of a flask, that looks like a genie coming out of a bottle.

Solved Name Date Lab Section Prelaboratory Problems Chegg Experiment 14 lab report on decomposition reaction rates course: general chemistry lab ii (che113) 408 documents university: university of kentucky. This demo makes use of the catalytic decomposition of hydrogen peroxide to produce a column of steam out of a flask, that looks like a genie coming out of a bottle. High school chemistry lab: decompose baking soda, apply stoichiometry to identify the correct reaction, and calculate percent yield. In part of this experiment, a decomposition reaction of a pure compound calcium oxide, cao, and carbon dioxide, co is analyzed. the masses of the pe compound and the calcium oxide are measured. Introduction a decomposition reaction is a type of chemical reaction where one reactant breaks down into two or more products. the general equation for this is ab → a b. Evidence for chemical reactions included color change, gas formation, and precipitate formation. the reactions demonstrated combination, decomposition, single displacement, and double displacement.
Comments are closed.