Solved Calculate The Concentration Of Unknown 1 And Unknown Chegg
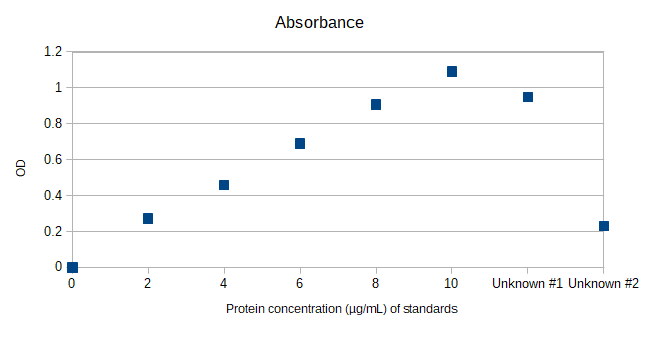
Solved Calculate The Concentration Of Unknown 1 And Unknown Chegg Answer according to graph concentration of solution one is 0.95 … not the question you’re looking for? post any question and get expert help quickly. Learning objectives calculate percentage concentration (m m, v v, m v), ppm and ppb. calculate the molarity of a solution. use concentration units to calculate the amount of solute in a solution. use molarity to determine quantities in chemical reactions. determine the resulting concentration of a diluted solution.
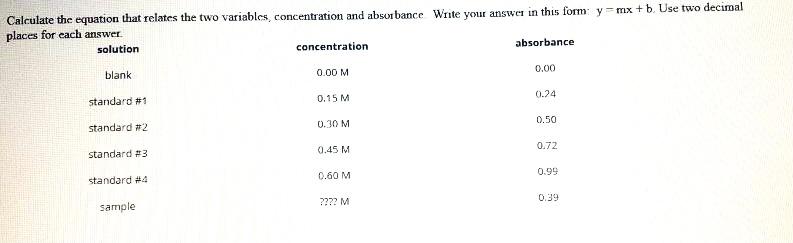
Solved Calculate The Unknown Concentration And Calculate The Chegg Use the concentration of the strong base and its volume added to find the moles. if your acid has one acidic hydrogen (monoprotic, if your teacher didn’t say then it’s probably monoprotic) then the moles of acid equals the moles of base. To calculate the concentration of an unknown solution, you typically use a process called titration. here's a general step by step guide: first, you need to prepare a solution of known concentration, called the titrant. then, you measure a specific volume of the unknown solution into a flask. Refractometry 7.1 to determine the concentration of an unknown solution of ethanol 7 standard mixtures of ethanol and water was prepared. the refractive index of these standards, water, pure ethanol and the unknown was determined. Step 1 3) the concentration of unknown (1)using the whole equation from the graph (2) can be calculated by: in th.
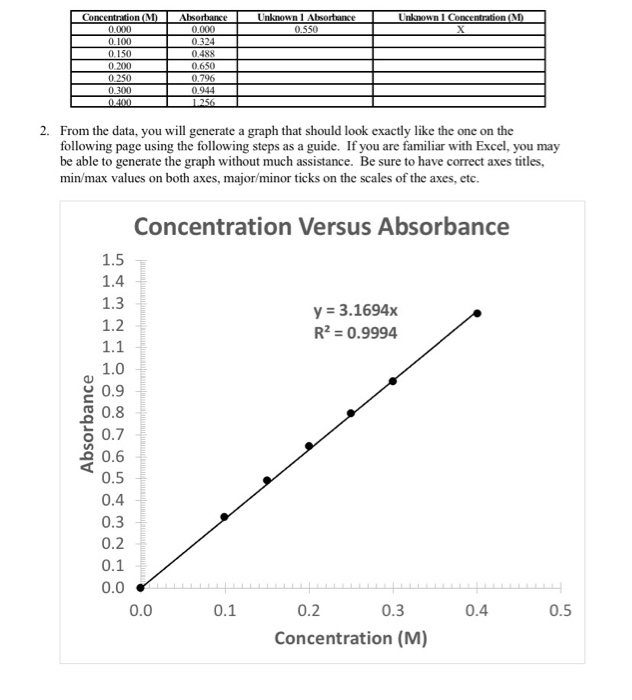
Solved Am I Able To Calculate Unknown 1 Adsorbance And Chegg Refractometry 7.1 to determine the concentration of an unknown solution of ethanol 7 standard mixtures of ethanol and water was prepared. the refractive index of these standards, water, pure ethanol and the unknown was determined. Step 1 3) the concentration of unknown (1)using the whole equation from the graph (2) can be calculated by: in th. The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line. To calculate the concentration of an unknown sample using a standard curve, you can follow these steps: create a standard curve: first, prepare a series of standard solutions with known concentrations of the substance of interest. First, we need to determine the absorbance of unknown 1 at 520 nm. from the data in experiment 3, we can see that the absorbance of unknown 1 at 520 nm is 0.45. In this case, dissolving 1 mol of (nh4)2cr2o7 produces a solution that contains 1 mol of cr2o72− ions and 2 mol of nh4 ions. (water molecules are omitted from a molecular view of the solution for clarity.).
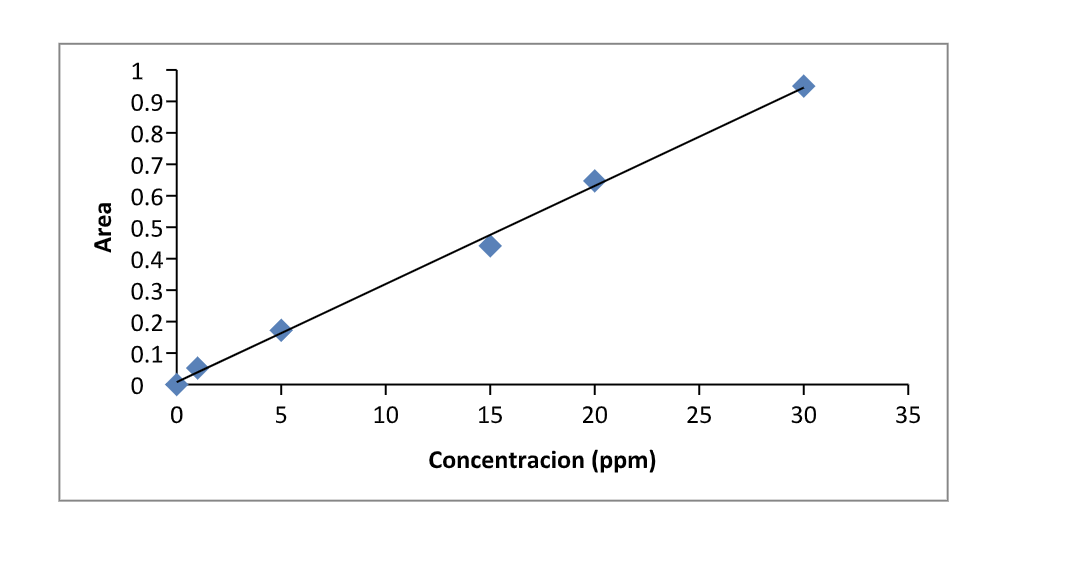
Solved Calculate The Concentration Of An Unknown Sample Chegg The absorbance of the unknown solution is used in conjunction with the calibration curve to determine the concentration of the analyte. the data obtained from the standard are used to plot a straight line. To calculate the concentration of an unknown sample using a standard curve, you can follow these steps: create a standard curve: first, prepare a series of standard solutions with known concentrations of the substance of interest. First, we need to determine the absorbance of unknown 1 at 520 nm. from the data in experiment 3, we can see that the absorbance of unknown 1 at 520 nm is 0.45. In this case, dissolving 1 mol of (nh4)2cr2o7 produces a solution that contains 1 mol of cr2o72− ions and 2 mol of nh4 ions. (water molecules are omitted from a molecular view of the solution for clarity.).
Comments are closed.