Solved Calculate The Concentration Of The Unknown Given The Chegg

Solved Calculate The Concentration Of The Unknown Given The Chegg Calculate the concentration of the unknown, given the equation below and a cell potential of 0.26 ag|ag* (x1)|| ag* (1.0m) ag. not the question you’re looking for? post any question and get expert help quickly. Use the concentration of the strong base and its volume added to find the moles. if your acid has one acidic hydrogen (monoprotic, if your teacher didn’t say then it’s probably monoprotic) then the moles of acid equals the moles of base.
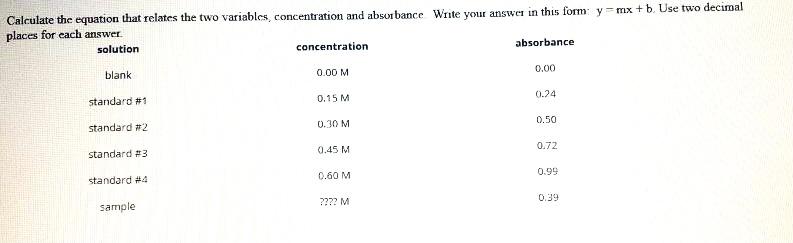
Solved Calculate The Unknown Concentration And Calculate The Chegg The concentration of an unknown niso4 solution is then determined by measuring its absorbance with the colorimeter. by locating the absorbance of the unknown on the vertical axis of the graph, the corresponding concentration can be found on the horizontal axis (follow the arrows in figure 2). To calculate the concentration of an unknown solution, you typically use a process called titration. here's a general step by step guide: first, you need to prepare a solution of known concentration, called the titrant. then, you measure a specific volume of the unknown solution into a flask. Calculate the molar refraction of water. the question does not provide the necessary data (n, mm, p) for water to calculate its molar refraction using the given formula. The definition of molarity can also be used to calculate a needed volume of solution, given its concentration and the number of moles desired, or the number of moles of solute (and subsequently, the mass of the solute), given its concentration and volume.

Solved Calculate The Concentration Of The Unknown Given The Chegg Calculate the molar refraction of water. the question does not provide the necessary data (n, mm, p) for water to calculate its molar refraction using the given formula. The definition of molarity can also be used to calculate a needed volume of solution, given its concentration and the number of moles desired, or the number of moles of solute (and subsequently, the mass of the solute), given its concentration and volume. First, we need to calculate the molar absorptivity (ε) of the unknown sample. since the molar absorptivity is a constant for a given substance, we can use the absorbance and concentration of the stock solution to calculate it. Calculate the concentration of an unknown solution in a concentration cell: close problem a concentration cell consisting of two hydrogen electrodes (pt) , where the cathode is a standard hydrogen electrode and the anode solution has an unknown pi, has a cell voltage of 0.252 v. Free practice questions for high school chemistry identifying unknown concentration. includes full solutions and score reporting. Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. substituting the given data.

Solved Calculate The Concentration Of The Unknown Naoh Then Chegg First, we need to calculate the molar absorptivity (ε) of the unknown sample. since the molar absorptivity is a constant for a given substance, we can use the absorbance and concentration of the stock solution to calculate it. Calculate the concentration of an unknown solution in a concentration cell: close problem a concentration cell consisting of two hydrogen electrodes (pt) , where the cathode is a standard hydrogen electrode and the anode solution has an unknown pi, has a cell voltage of 0.252 v. Free practice questions for high school chemistry identifying unknown concentration. includes full solutions and score reporting. Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. substituting the given data.
Comments are closed.