Solved A Chemist Is Tasked With Determining The Percentage Chegg

Solved A Chemist Is Tasked With Determining The Percentage Chegg Here’s the best way to solve it. identify the reactants and products and make sure the chemical equation for the reaction between hydrochloric acid (hcl) and calcium hydroxide (ca (oh)2) is balanced. a chemist is tasked with determining the percentage purity of a sample of sodium carbonate. A chemist is tasked with determining the percentage purity of a sample of sodium carbonate. to do this, 0.60 g of the sample was dissolved in enough water to make 250.0 ml of solution.
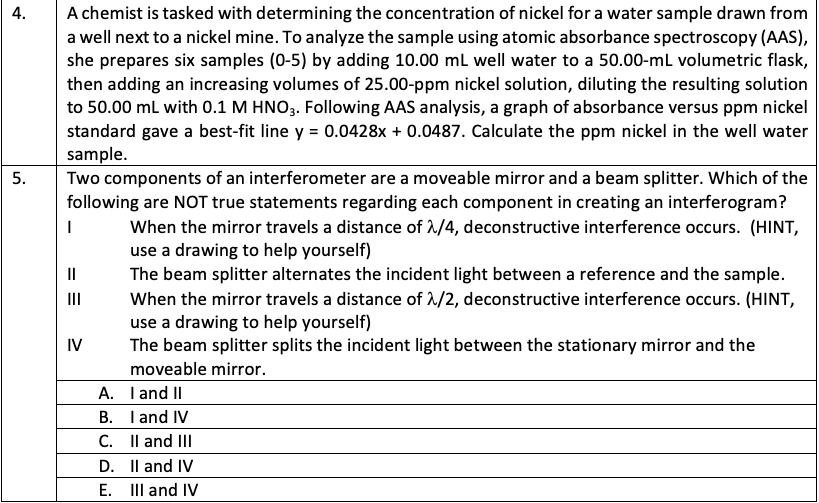
Solved 4 5 A Chemist Is Tasked With Determining The Chegg 4.4.1: practice problems formula mass, percent composition, and the mole is shared under a not declared license and was authored, remixed, and or curated by libretexts. A chemist is tasked with determining the concentration of chloride ions (cl ) in a water sample collected from a local river. the chemist decides to use gravimetric analysis to accomplish this task. To determine the acetic acid concentration in vinegar samples, you will need to use a standardization procedure. the standardization procedure that you have done the day before involved consuming 0.8956 of pure khp. today, you will dilute a vinegar sample to the mark with distilled water. A useful analysis of brass involves the determination of its copper content. this inboratory experiment provides a straightforward method for determining the percent of copper in a brass sample, as explained below.

Solved You Are Tasked With Determining The Mass Percentage Chegg To determine the acetic acid concentration in vinegar samples, you will need to use a standardization procedure. the standardization procedure that you have done the day before involved consuming 0.8956 of pure khp. today, you will dilute a vinegar sample to the mark with distilled water. A useful analysis of brass involves the determination of its copper content. this inboratory experiment provides a straightforward method for determining the percent of copper in a brass sample, as explained below. Stuck on a stem question? post your question and get video answers from professional experts: step 1: understand the concept of percent composition before. To determine the concentration of nickel in the well water sample, we need to use the equation of the best fit line obtained from the aas analysis. this line represents the calibration curve for the aas instrument, which relates the absorbance of the sample to the concentration of nickel. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess hydrochloric acid and then precipitating the silver as agcl. Designed for learning we trained chegg’s ai tools using our own step by step homework solutions–you’re not just getting an answer, you’re learning how to solve the problem.

Solved Percentage Chegg Stuck on a stem question? post your question and get video answers from professional experts: step 1: understand the concept of percent composition before. To determine the concentration of nickel in the well water sample, we need to use the equation of the best fit line obtained from the aas analysis. this line represents the calibration curve for the aas instrument, which relates the absorbance of the sample to the concentration of nickel. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess hydrochloric acid and then precipitating the silver as agcl. Designed for learning we trained chegg’s ai tools using our own step by step homework solutions–you’re not just getting an answer, you’re learning how to solve the problem.

Solved Show Your Solution 2 Problem You Were Tasked To Chegg A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess hydrochloric acid and then precipitating the silver as agcl. Designed for learning we trained chegg’s ai tools using our own step by step homework solutions–you’re not just getting an answer, you’re learning how to solve the problem.

Solved Four Chemists Are Asked To Determine The Percentage Chegg
Comments are closed.