Solved 18 The Standard Emf For The Cell Using The Overall Chegg
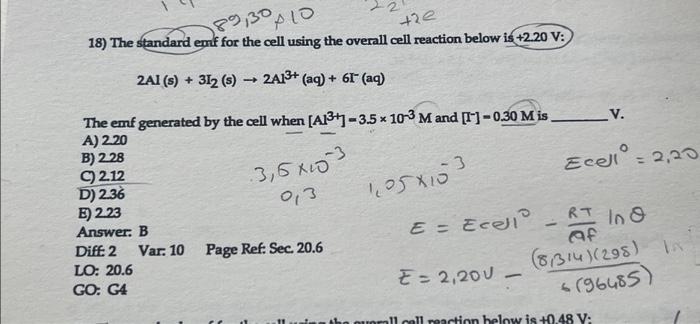
Solved 18 The Standard Emf For The Cell Using The Overall Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. not the question you’re looking for? post any question and get expert help quickly. The nernst equation is a well established formula in electrochemistry used to determine the potential of a cell under non standard conditions, reflecting changes in concentrations of reactants and products.

Solved The Standard Emf For The Cell Using The Overall Cell Chegg To solve this problem, we can use the nernst equation, which relates the reduction potential of an electrochemical reaction (i.e., the voltage or emf) to the standard electrode. The standard emf for the cell using the overall cell reaction below is 0.48 v: zn (s) ni2 (aq) → zn2 (aq) ni (s). the emf generated by the cell when [ni2 ] = 0.100 m and [zn2 ] = 2.25 m is ?. Value of standard change in gibbs free energy Δ g o = 405.3 kj. which explanation given below. Question: 18) the standard emf for the cell using the overall cell reaction below is 2.20 v : 2ai (s) 3i2 ( s)→2al3 (aq) 6i (aq) the emf generated by the cell when [al3 ]=3.5×10−3m and [i−]=0.30m is v.

Solved The Standard Emf For The Cell Using The Overall Cell Chegg Value of standard change in gibbs free energy Δ g o = 405.3 kj. which explanation given below. Question: 18) the standard emf for the cell using the overall cell reaction below is 2.20 v : 2ai (s) 3i2 ( s)→2al3 (aq) 6i (aq) the emf generated by the cell when [al3 ]=3.5×10−3m and [i−]=0.30m is v. To determine the cell potential (emf) under non standard conditions, we use the nernst equation. the nernst equation allows us to calculate the cell potential when concentrations of ionic species are not at standard conditions (1 m concentration). To solve this problem, we can use the nernst equation, which relates the reduction potential of an electrochemical reaction (i.e., the voltage or emf) to the standard electrode potential, temperature, and the reaction quotient q. Fluorine 18 undergoes positron emission with a half life of 1.10 × 10^2 minutes. if a patient is given a 248 mg dose for a pet scan, how long will it take for the amount of fluorine 18 to drop to 83 mg? (assume that none of the fluorine is excreted from the body.). Calculate the standard emf of a cell that uses the mg mg2 m g m g 2 and cu cu2 c u c u 2 half cell reactions at 25∘c 25 ∘ c. write the equation for the cell reaction that occurs under standard state conditions.
Comments are closed.