Quizartinib Improves Overall Survival In Adult Patients With Flt3 Itd

Quizartinib Improves Overall Survival In Adult Patients With Flt3 Itd Interpretation: the addition of quizartinib to standard chemotherapy with or without allo hct, followed by continuation monotherapy for up to 3 years, resulted in improved overall survival in adults aged 18 75 years with flt3 itd positive newly diagnosed aml. The addition of quizartinib to standard chemotherapy and consolidation significantly improves overall survival (os) in adults with newly diagnosed flt3 itd acute myeloid leukemia (aml), according to final results of the phase 3 quantum first trial.

Quizartinib In Flt3 Itd Positive Aml Patient Empowerment Network “adding targeted treatment with quizartinib, a potent and selective flt3 inhibitor, to standard chemotherapy resulted in a doubling of median overall survival in patients with newly diagnosed flt3 itd positive acute myeloid leukemia compared to standard chemotherapy alone. An earlier phase 3 study found that quizartinib, given as a single agent, improved survival in patients with relapsed refractory flt3 itd positive aml compared with standard of care. The addition of quizartinib to standard chemotherapy with or without allo hct, followed by continuation monotherapy for up to 3 years, resulted in improved overall survival in adults aged 18–75 years with flt3 itd positive newly diagnosed aml. These findings are in line with the u.s. food and drug administration’s recent approval of quizartinib in combination with standard induction and consolidation chemotherapy, followed by continuation monotherapy, for adult patients with newly diagnosed, flt3 itd positive aml.
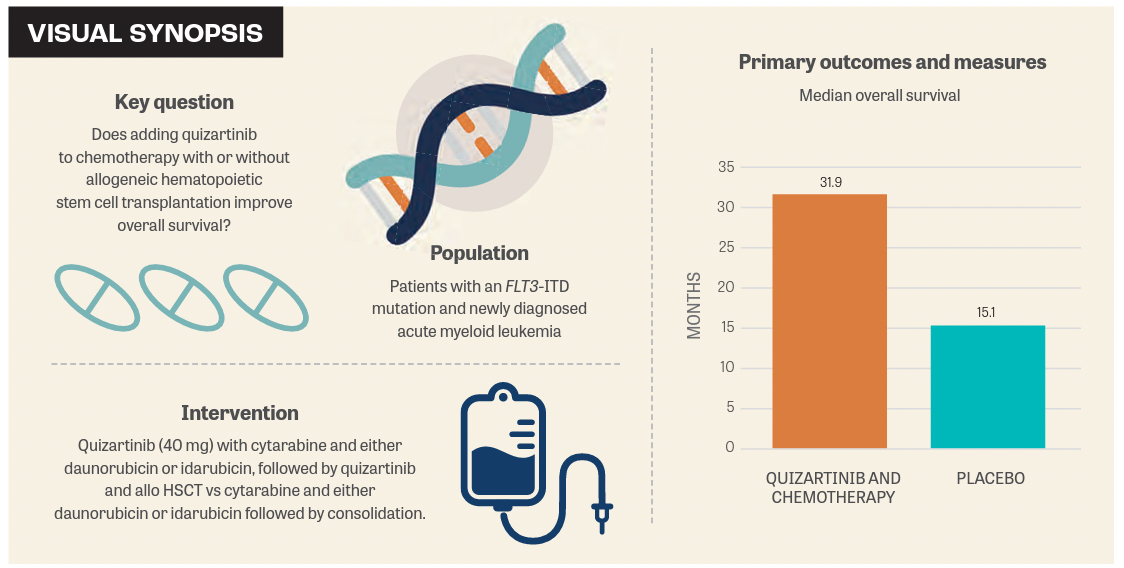
Quizartinib Improves Os In Flt3 Itd Aml The addition of quizartinib to standard chemotherapy with or without allo hct, followed by continuation monotherapy for up to 3 years, resulted in improved overall survival in adults aged 18–75 years with flt3 itd positive newly diagnosed aml. These findings are in line with the u.s. food and drug administration’s recent approval of quizartinib in combination with standard induction and consolidation chemotherapy, followed by continuation monotherapy, for adult patients with newly diagnosed, flt3 itd positive aml. Although clinically active, first generation flt3 inhibitors had limited success as single agents. this led to the development of a second generation of more selective flt3 inhibitors. this review focuses on quizartinib, a potent second generation flt3 inhibitor. The addition of quizartinib to standard chemotherapy and up to 3 years of continuation therapy more than doubled overall survival in adults with newly diagnosed flt3 itd–positive aml vs the standard of care (31.9 vs 15.1 months). The novel precision cancer medicine, vanflyta (quizartinib) improves the survival of individuals with relapsed refractory acute myeloid leukemia (aml) with flt3 itd mutations when compared to standard salvage chemotherapy. 1. Management paradigms for newly diagnosed acute myeloid leukemia (nd aml) in patients considered unfit to receive intensive chemotherapy have evolved with improved understanding of disease biology.
Quizartinib Improves Os In Multiple Flt3 Itd Aml Subgroups Although clinically active, first generation flt3 inhibitors had limited success as single agents. this led to the development of a second generation of more selective flt3 inhibitors. this review focuses on quizartinib, a potent second generation flt3 inhibitor. The addition of quizartinib to standard chemotherapy and up to 3 years of continuation therapy more than doubled overall survival in adults with newly diagnosed flt3 itd–positive aml vs the standard of care (31.9 vs 15.1 months). The novel precision cancer medicine, vanflyta (quizartinib) improves the survival of individuals with relapsed refractory acute myeloid leukemia (aml) with flt3 itd mutations when compared to standard salvage chemotherapy. 1. Management paradigms for newly diagnosed acute myeloid leukemia (nd aml) in patients considered unfit to receive intensive chemotherapy have evolved with improved understanding of disease biology.
Comments are closed.