Process Validation Pdf Verification And Validation Life Cycle

Process Validation Life Cycle Approach Catherine Neary Gmp Inspector A true life cycle approach to process validation requires gathering useful, scientific information as process and control strategies are developed, thus saving time and resources during later qualification and validation stages. This discussion paper is focused on the challenges of implementing a current process validation lifecycle approach in the quality management of a set of products initially validated and commercialized prior to the formal introduction of the current lifecycle architecture.

Validation Life Cycle Process validation involves a series of activities taking place over the lifecycle of the product and process. this guidance describes the process validation activities in three stages. Verification was the end product realized right? validation was the right end product realized? flexibility is allowed in the timing, number, and content of reviews as long as the equivalent information is provided at each kdp and the approach is fully documented in the project plan. The document discusses process validation which ensures a process is capable of consistently producing quality products. it covers process design, qualification, and continued verification throughout a product's life cycle. Both the united states and the european union offer guidance on a life cycle approach to process validation. this goes beyond the traditional three to five lots.

Validation For Compliance Information And Training Presentation The document discusses process validation which ensures a process is capable of consistently producing quality products. it covers process design, qualification, and continued verification throughout a product's life cycle. Both the united states and the european union offer guidance on a life cycle approach to process validation. this goes beyond the traditional three to five lots. Es how the modern concept for process validation (6,7), which is based on a lifecycle model, can be applied to analytical procedures (8–11). we propose that the traditional approaches to validation, transfer, and verification should be integrated into the analytical procedure lifecy. This ispe good practice guide: practical implementation of the lifecycle approach to process validation provides detailed practical guidance to help pharmaceutical companies meet global regulatory process validation expectations. In the following sections, we describe general considerations for process validation, the recommended stages of process validation, and specific activities for each stage in the product lifecycle. The objective of this review provide an introduction and a general overview of the process validation of pharmaceutical production with specific reference to the criteria lay down by the us fda.
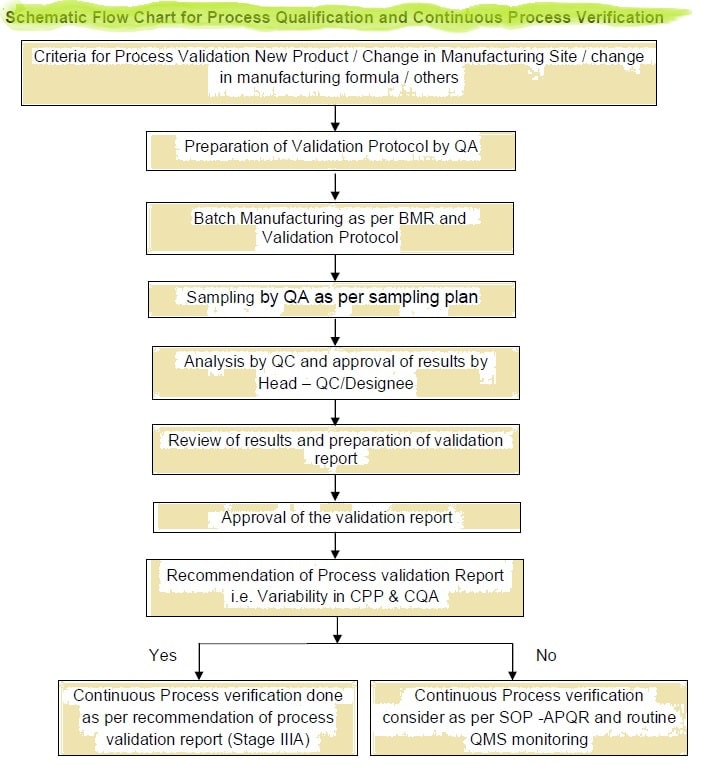
Process Validation Pv Verification Of Drug Product Guidelines Sops Es how the modern concept for process validation (6,7), which is based on a lifecycle model, can be applied to analytical procedures (8–11). we propose that the traditional approaches to validation, transfer, and verification should be integrated into the analytical procedure lifecy. This ispe good practice guide: practical implementation of the lifecycle approach to process validation provides detailed practical guidance to help pharmaceutical companies meet global regulatory process validation expectations. In the following sections, we describe general considerations for process validation, the recommended stages of process validation, and specific activities for each stage in the product lifecycle. The objective of this review provide an introduction and a general overview of the process validation of pharmaceutical production with specific reference to the criteria lay down by the us fda.
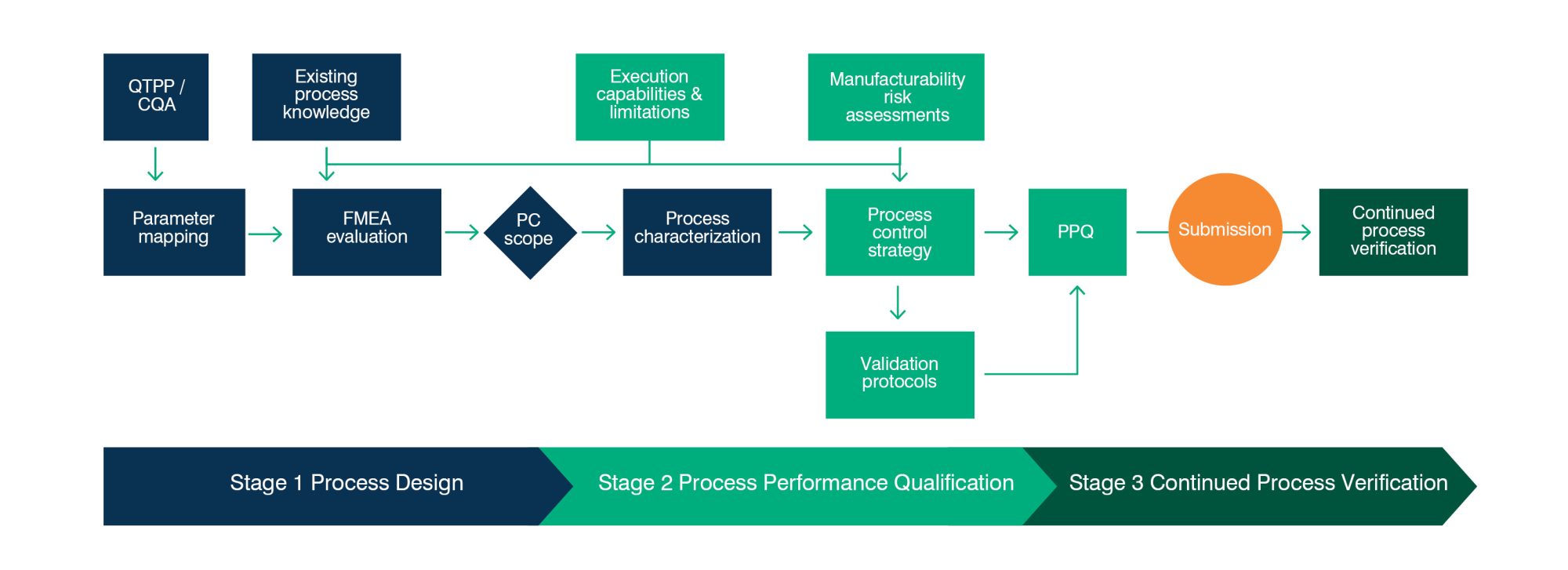
Continued Process Verification In The Process Validat Vrogue Co In the following sections, we describe general considerations for process validation, the recommended stages of process validation, and specific activities for each stage in the product lifecycle. The objective of this review provide an introduction and a general overview of the process validation of pharmaceutical production with specific reference to the criteria lay down by the us fda.

The Validation Life Cycle Download Scientific Diagram
Comments are closed.