New Targets To Treat Acute Myeloid Leukemia Aml With Flt3 Mutations

Flt3 Mutations In Acute Myeloid Leukemia Aml Biorender Science The understanding of the molecular pathobiology of acute myeloid leukemia (aml) has spurred the identification of therapeutic targets and the development of corresponding novel. On july 20, the agency approved quizartinib (vanflyta) combined with chemotherapy as part of the initial treatment of people with aml that has a specific change in a gene called flt3. genetic changes in flt3 are common in people diagnosed with aml.
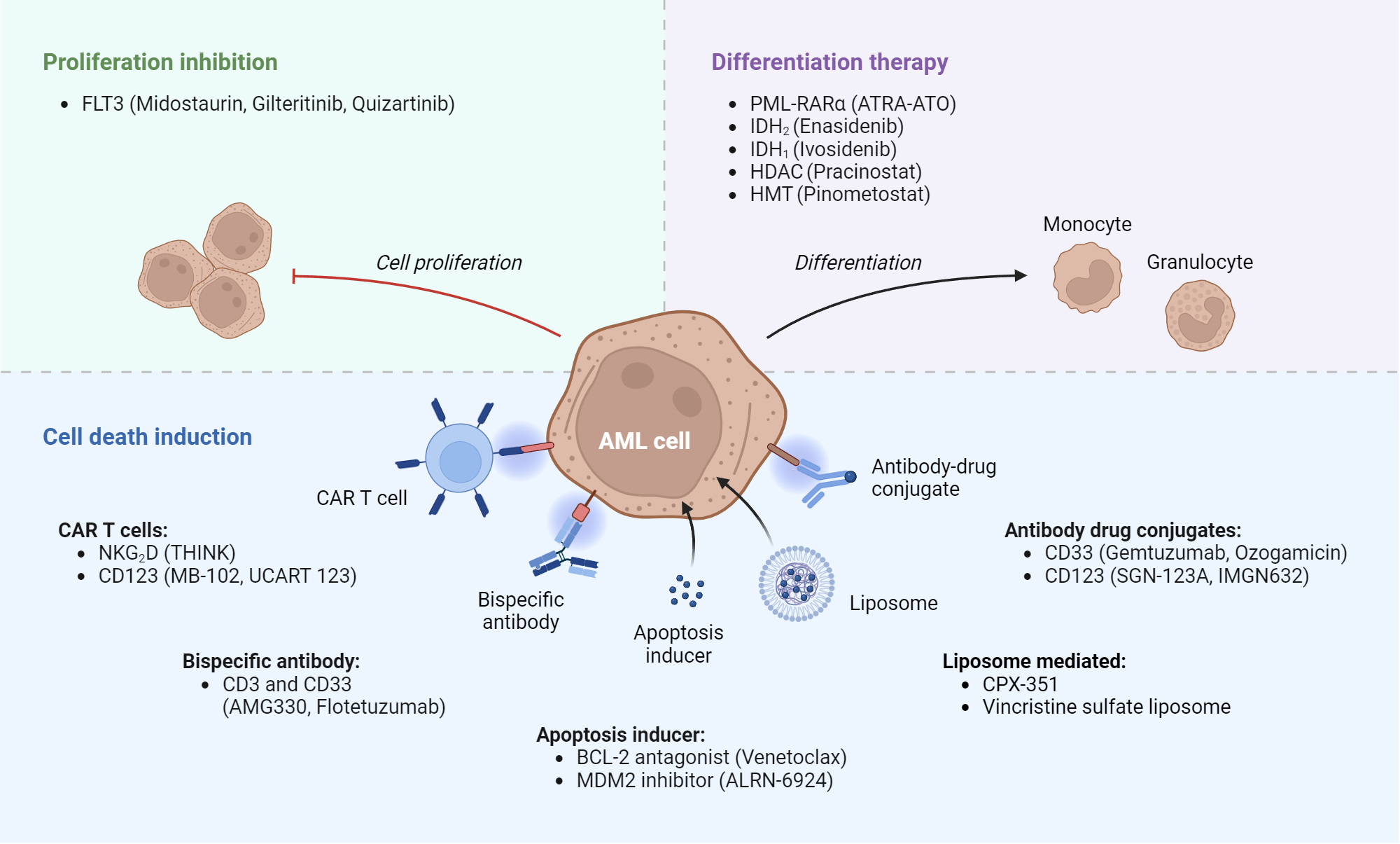
Acute Myeloid Leukemia Targets And Therapies Biorender Science Templates This review seeks to identify and describe novel genetic and protein targets and their associated therapeutics currently being used or studied in the treatment of acute myeloid leukemia (aml). Focused research in acute myeloid leukemia (aml) biology and treatment has led to the identification of new therapeutic targets and several new drug approvals over the last decade. progressive improvements in response and survival have mirrored these improvements in treatment options. Rye brook, n.y., july 25, 2023 – the u.s. food and drug administration (fda) last week approved quizartinib (vanflyta®) in combination with standard chemotherapies for the treatment of adult patients with newly diagnosed acute myeloid leukemia (aml) that carries a genetic mutation called flt3 itd. Midostaurin (rydapt) and quizartinib (vanflyta) are flt3 inhibitors that can be used along with certain chemotherapy drugs to treat newly diagnosed adults whose leukemia cells have a mutation in the flt3 gene.
Acute Myeloid Leukemia Plos One Rye brook, n.y., july 25, 2023 – the u.s. food and drug administration (fda) last week approved quizartinib (vanflyta®) in combination with standard chemotherapies for the treatment of adult patients with newly diagnosed acute myeloid leukemia (aml) that carries a genetic mutation called flt3 itd. Midostaurin (rydapt) and quizartinib (vanflyta) are flt3 inhibitors that can be used along with certain chemotherapy drugs to treat newly diagnosed adults whose leukemia cells have a mutation in the flt3 gene. New targeted drugs have emerged, including venetoclax to target b cell lymphoma 2, midostaurin and gilteritinib to target flt3, and ivosidenib and enasidenib to target mutant isocitrate dehydrogenase 1 and 2, respectively. Background there is no established standard treatment for acute myeloid leukemia (aml) patients with flt3 wild type relapsing after allogeneic hematopoietic stem cell transplantation (allo hsct). multi kinase inhibitor sorafenib has been widely explored in the treatment of aml patients with flt3 internal tandem duplication (flt3 itd) mutations. some studies have revealed that the addition of. Venetoclax based regimens in aml: the new standard or a temporary fix? abstract acute myeloid leukemia (aml)remains a challenginghematologic malignancy, particularly among older adults and patients unfit for intensive chemotherapy. over the past decade, treatment models have shifted with the advent of venetoclax, a selective bcl 2 inhibitor, in combination with hypomethylating agents. this. The second generation flt3 inhibitor gilteritinib was approved for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (aml) with an flt3 mutation, as detected by an fda approved test.

Case Study Presents 3 Potential Novel Targets In Acute Myeloid Leukemia New targeted drugs have emerged, including venetoclax to target b cell lymphoma 2, midostaurin and gilteritinib to target flt3, and ivosidenib and enasidenib to target mutant isocitrate dehydrogenase 1 and 2, respectively. Background there is no established standard treatment for acute myeloid leukemia (aml) patients with flt3 wild type relapsing after allogeneic hematopoietic stem cell transplantation (allo hsct). multi kinase inhibitor sorafenib has been widely explored in the treatment of aml patients with flt3 internal tandem duplication (flt3 itd) mutations. some studies have revealed that the addition of. Venetoclax based regimens in aml: the new standard or a temporary fix? abstract acute myeloid leukemia (aml)remains a challenginghematologic malignancy, particularly among older adults and patients unfit for intensive chemotherapy. over the past decade, treatment models have shifted with the advent of venetoclax, a selective bcl 2 inhibitor, in combination with hypomethylating agents. this. The second generation flt3 inhibitor gilteritinib was approved for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (aml) with an flt3 mutation, as detected by an fda approved test.

Acute Myeloid Leukemia Diagnosis Prognosis Treatment And Outcomes Venetoclax based regimens in aml: the new standard or a temporary fix? abstract acute myeloid leukemia (aml)remains a challenginghematologic malignancy, particularly among older adults and patients unfit for intensive chemotherapy. over the past decade, treatment models have shifted with the advent of venetoclax, a selective bcl 2 inhibitor, in combination with hypomethylating agents. this. The second generation flt3 inhibitor gilteritinib was approved for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (aml) with an flt3 mutation, as detected by an fda approved test.
New Targeted Therapy Bolsters Standard Treatment For Acute Myeloid
Comments are closed.