Need Of A Three Electrode System
Three Electrode System Openclipart The article explains the three electrode system used in electrochemical research. this setup allows precise control and measurement of electrochemical reactions, providing accurate results compared to the traditional two electrode method. For high sensitivity measurements, studies of charge transfer processes, or in depth analysis of electrode reaction mechanisms, the three electrode system is virtually indispensable. the working electrode is the core of the electrochemical cell—it’s where the reaction of interest occurs.

Three Electrode Cell Mono Mole Three electrodes system can employ because the counter electrode is required to prevent current from flowing through the reference electrode, which would alter the potential of the reference. The three electrode system consists of a working electrode, counter electrode, and reference electrode. the reference electrode’s role is to act as a reference in measuring and controlling the working electrode potential, without passing any current. Discover the essentials of three electrode systems in battery testing. learn how to apply and customize these systems to enhance electrochemical analysis, improve testing precision, and optimize battery research processes. Three electrode setups have a distinct experimental advantage over two electrode setups: they measure only one half of the cell. that is, the potential changes of the working electrode are measured independent of changes that may occur at the counter electrode.

Three Electrode System 1 Test Sample Working Electrode 2 Sce Discover the essentials of three electrode systems in battery testing. learn how to apply and customize these systems to enhance electrochemical analysis, improve testing precision, and optimize battery research processes. Three electrode setups have a distinct experimental advantage over two electrode setups: they measure only one half of the cell. that is, the potential changes of the working electrode are measured independent of changes that may occur at the counter electrode. As the solid state battery field matures and more attention is turned to detailed characterization of interfaces and individual redox processes, the need for a suitable reference electrode (re) system emerges, in order to enable three electrode measurements [1, 2, 3]. During three electrode experiments, charge flow (current) primarily occurs between the working electrode and the counter electrode while the potential of the working electrode is measured with respect to the reference electrode. Why do you need three electrodes system and a supporting electrolyte in voltametric experiments? large currents passing through an electrode can change its potential. therefore, if you want careful control and measurement of both potential and current through a cell, you want to use three electrodes. In the development and production of batteries, the three electrode test system is an important analytical tool for accurately measuring and analyzing the electrochemical properties of.

Preparation Flow Chart A Three Electrode System B Two Electrode System As the solid state battery field matures and more attention is turned to detailed characterization of interfaces and individual redox processes, the need for a suitable reference electrode (re) system emerges, in order to enable three electrode measurements [1, 2, 3]. During three electrode experiments, charge flow (current) primarily occurs between the working electrode and the counter electrode while the potential of the working electrode is measured with respect to the reference electrode. Why do you need three electrodes system and a supporting electrolyte in voltametric experiments? large currents passing through an electrode can change its potential. therefore, if you want careful control and measurement of both potential and current through a cell, you want to use three electrodes. In the development and production of batteries, the three electrode test system is an important analytical tool for accurately measuring and analyzing the electrochemical properties of.

The Classical Three Electrode System Download Scientific Diagram Why do you need three electrodes system and a supporting electrolyte in voltametric experiments? large currents passing through an electrode can change its potential. therefore, if you want careful control and measurement of both potential and current through a cell, you want to use three electrodes. In the development and production of batteries, the three electrode test system is an important analytical tool for accurately measuring and analyzing the electrochemical properties of.

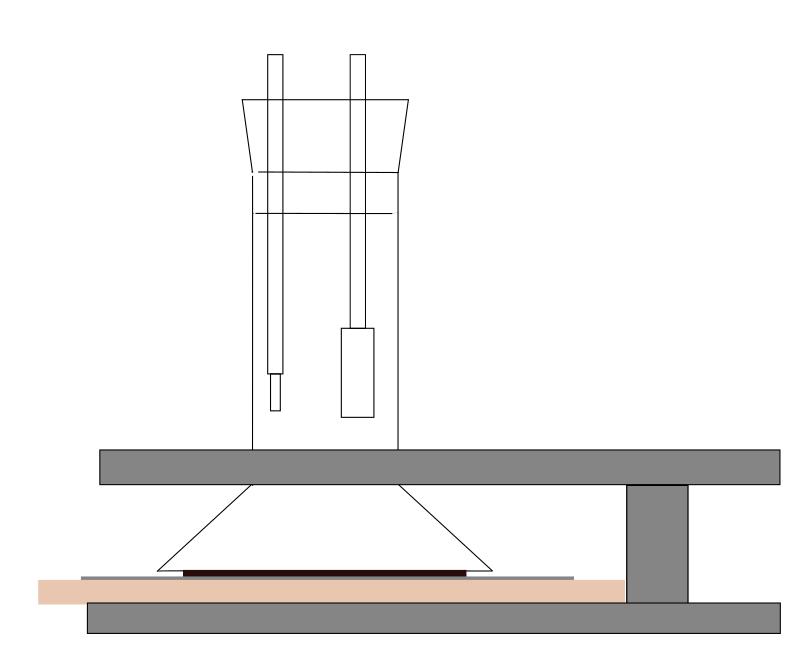
Setup For Three Electrode System Download Scientific Diagram
Comments are closed.