Implementing A Compliant Solution For The Electronic Distribution Of

Developing Implementing Distribution Strategies Pdf Supply Chain It clearly takes a significant effort to implement an electronic system for the distribution of ifu that complies with the different regulatory, functional and operational requirements. Edi compliance mandates are rules set by individual retailers to streamline and standardize how they communicate with their supplier network. each retailer’s requirements are unique, based on the edi transactions they use—like shipping notices or invoices—and how quickly they expect to receive them.

Implementing A Compliant Solution For The Electronic Distribution Of In return, software users need a dependable way to quickly send log files and corrupt databases or software back to the company for diagnosis. businesses also require an efficient process to track, audit and report software distribution for revenue recognition, entitlement and compliance purposes. Fda has issued the following guidance and policy documents since the drug supply chain security act (dscsa) was enacted in 2013. visit fda’s implementation of dscsa requirements and public health. Various regulatory frameworks play a critical role in driving the adoption of electronic distribution solutions within pharmaceutical supply chains. one of the key regulatory drivers is the drug supply chain security act (dscsa) enacted in the united states. Simplerqms helps life science companies to achieve 21 cfr part 11 compliance by providing a comprehensive eqms software solution that facilitates the management of electronic records, signatures, and quality documentation.

Distribution Solution At Rs 5280 Year Jaipur Id 2849964106273 Various regulatory frameworks play a critical role in driving the adoption of electronic distribution solutions within pharmaceutical supply chains. one of the key regulatory drivers is the drug supply chain security act (dscsa) enacted in the united states. Simplerqms helps life science companies to achieve 21 cfr part 11 compliance by providing a comprehensive eqms software solution that facilitates the management of electronic records, signatures, and quality documentation. Euro american worldwide logistics offers expertise in dscsa compliance solutions – from turnkey serialization systems to blockchain enabled track and trace platforms. our team can assess your current compliance status and help implement robust, future proof logistics tracking. Regulatory compliance: the automated and standardized approach to software deployment facilitated by electronic software distribution ensures adherence to regulatory compliance, thereby averting potential penalties and reputational damage. Create an efficient global labeling strategy that is compliant with both electronic and paper based package insert requirements. Learn what fda 21 cfr part 11 is, why it matters, and how to ensure compliance. explore software validation, electronic records, and audit trails.

Electronic Distribution Pptx Euro american worldwide logistics offers expertise in dscsa compliance solutions – from turnkey serialization systems to blockchain enabled track and trace platforms. our team can assess your current compliance status and help implement robust, future proof logistics tracking. Regulatory compliance: the automated and standardized approach to software deployment facilitated by electronic software distribution ensures adherence to regulatory compliance, thereby averting potential penalties and reputational damage. Create an efficient global labeling strategy that is compliant with both electronic and paper based package insert requirements. Learn what fda 21 cfr part 11 is, why it matters, and how to ensure compliance. explore software validation, electronic records, and audit trails.
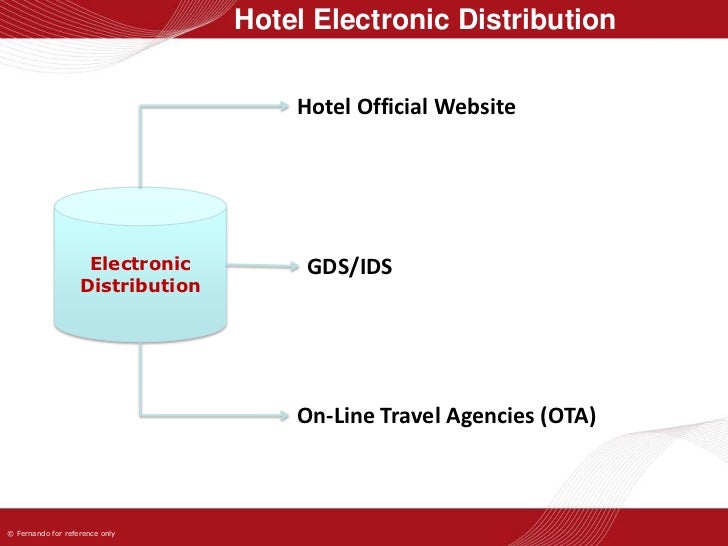
Electronic Distribution Create an efficient global labeling strategy that is compliant with both electronic and paper based package insert requirements. Learn what fda 21 cfr part 11 is, why it matters, and how to ensure compliance. explore software validation, electronic records, and audit trails.
Comments are closed.