Drug Recommendation System Based On Sentiment Analysis Of Drug Reviews Using Machine Learning

Drug Recommendation System Based On Sentiment Analysis Of Drug Reviews The use of psychoactive drugs without medical supervision is associated with significant health risks and can lead to the development of drug use disorders. drug use disorders, particularly when untreated, increase morbidity and mortality risks for individuals, can trigger substantial suffering and lead to impairment in personal, family, social, educational, occupational or other important. About who drug information who drug information is a quarterly journal providing an overview of topics relating to medicines development and regulation which is targeted to a wide audience of health professionals and policy makers. launched in 1987, who drug information communicates the latest international news and trends to regulatory agencies, academic and training institutions, researchers.
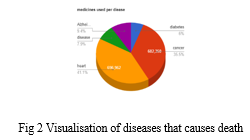
Drug Recommendation System Based On Sentiment Analysis Of Drug Reviews A new report from the world health organization (who) highlights that 2.6 million deaths per year were attributable to alcohol consumption, accounting for 4.7% of all deaths, and 0.6 million deaths to psychoactive drug use. notably, 2 million of alcohol and 0.4 million of drug attributable deaths were among men. The unit works globally to improve health and well being of populations by articulating, promoting, supporting and monitoring evidence informed policies, strategies and interventions to reduce the burden associated with alcohol, drugs and addictive behaviours. Who announces development of updated guidelines for the psychosocially assisted pharmacological treatment of opioid dependence and community management of opioid overdosein 2022, approximately 60 million people globally engaged in non medical opioid use, including the use of drugs like heroin, morphine, codeine, fentanyl, methadone, tramadol, and other similar substances. their regular non. Module 4: treatment and care encompass all current recommendations for managing drug susceptible and drug resistant tb, alongside patient care and support strategies. developed according to who’s rigorous standards, the guidelines rely on the latest evidence reviews and the grade methodology to evaluate evidence quality and determine the strength of each recommendation. primarily aimed at.

Drug Recommendation System Based On Sentiment Analysis Of Drug Reviews Who announces development of updated guidelines for the psychosocially assisted pharmacological treatment of opioid dependence and community management of opioid overdosein 2022, approximately 60 million people globally engaged in non medical opioid use, including the use of drugs like heroin, morphine, codeine, fentanyl, methadone, tramadol, and other similar substances. their regular non. Module 4: treatment and care encompass all current recommendations for managing drug susceptible and drug resistant tb, alongside patient care and support strategies. developed according to who’s rigorous standards, the guidelines rely on the latest evidence reviews and the grade methodology to evaluate evidence quality and determine the strength of each recommendation. primarily aimed at. 1.1 scope of the document 1.1.1 e guidance contained in this document is intended to provide information about the available specications for water for pharmaceutical use (wpu), guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical ingredients (apis) and dosage forms, and to provide guidance on good manufacturing practices (gmp. The world health organization (who) has officially designated health canada, the ministry of health, labour and welfare pharmaceuticals and medical devices agency (mhlw pmda) of japan, and the medicines and healthcare products regulatory agency (mhra) of the united kingdom as who listed authorities (wlas), a status granted to national authorities that meet the highest international regulatory. 1. introduction and background manufacturers should ensure that the products that they manufacture are safe, efective and of the quality required for their intended use. systems should be in place to ensure that pharmaceutical products are produced according to validated processes and to defined procedures. manufacturing processes should be shown to be capable of consistently manufacturing. Guidance for industry. sterile drug products produced by aseptic processing. japan, 2005. manufacture of sterile medicinal products. in: the rules governing medicinal products in the european union vol. 4. eu guidelines to good manufacturing practice medicinal products for human and veterinary use. annex 1, brussels, 2008.

System For Recommending Drugs Based On Machine Learning Sentiment 1.1 scope of the document 1.1.1 e guidance contained in this document is intended to provide information about the available specications for water for pharmaceutical use (wpu), guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical ingredients (apis) and dosage forms, and to provide guidance on good manufacturing practices (gmp. The world health organization (who) has officially designated health canada, the ministry of health, labour and welfare pharmaceuticals and medical devices agency (mhlw pmda) of japan, and the medicines and healthcare products regulatory agency (mhra) of the united kingdom as who listed authorities (wlas), a status granted to national authorities that meet the highest international regulatory. 1. introduction and background manufacturers should ensure that the products that they manufacture are safe, efective and of the quality required for their intended use. systems should be in place to ensure that pharmaceutical products are produced according to validated processes and to defined procedures. manufacturing processes should be shown to be capable of consistently manufacturing. Guidance for industry. sterile drug products produced by aseptic processing. japan, 2005. manufacture of sterile medicinal products. in: the rules governing medicinal products in the european union vol. 4. eu guidelines to good manufacturing practice medicinal products for human and veterinary use. annex 1, brussels, 2008.

Drug Recommender System Using Machine Learning For Sentiment Analysis 1. introduction and background manufacturers should ensure that the products that they manufacture are safe, efective and of the quality required for their intended use. systems should be in place to ensure that pharmaceutical products are produced according to validated processes and to defined procedures. manufacturing processes should be shown to be capable of consistently manufacturing. Guidance for industry. sterile drug products produced by aseptic processing. japan, 2005. manufacture of sterile medicinal products. in: the rules governing medicinal products in the european union vol. 4. eu guidelines to good manufacturing practice medicinal products for human and veterinary use. annex 1, brussels, 2008.
Comments are closed.