Difference Between Static And Dynamic Equilibrium Pediaa Com
Static Equilibrium Archives Pediaa Com In a dynamic equilibrium, changes occur within the mixture, keeping the total composition the same. in a static equilibrium, there are no further changes taking place within the mixture. While both dynamic equilibrium and static equilibrium involve a state of balance, there are several key differences between the two: dynamic equilibrium involves continuous change and movement, while static equilibrium is characterized by stability and motionlessness.

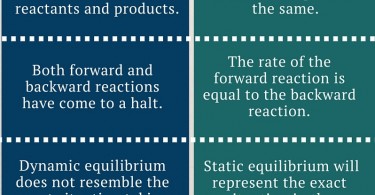
Difference Between Static And Dynamic Equilibrium Although a body in dynamic equilibrium may seem at rest, it’s actually in motion. in contrast, a body in static equilibrium remains at rest with an applied external force. the major difference between static and dynamic equilibrium is the state of the body when all the forces are acting upon it. At static equilibrium, there are no dynamic forces acting on the reactants nor the products. as a result, at the point of equilibrium the reaction practically stops and there are no movements between reactants and products. Static equilibrium is an equilibrium that occurs when all particles in the reaction are at rest and there is no motion between reactants and products. dynamic equilibrium is an equilibrium that occurs when the rate of formation of product and the rate of decay of product back to reactant is same. Difference between static and dynamic equilibrium static equilibrium refers to a condition where the reaction occurring in a system is completely halted, and there exists no movement between the reactants and the products corresponding to the chemical reaction.

Difference Between Static And Dynamic Equilibrium Static equilibrium is an equilibrium that occurs when all particles in the reaction are at rest and there is no motion between reactants and products. dynamic equilibrium is an equilibrium that occurs when the rate of formation of product and the rate of decay of product back to reactant is same. Difference between static and dynamic equilibrium static equilibrium refers to a condition where the reaction occurring in a system is completely halted, and there exists no movement between the reactants and the products corresponding to the chemical reaction. Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction. static equilibrium, also known as mechanical equilibrium, means the reaction has stopped. in other words, the system is at rest. Static equilibrium represents a state of rest where forces are perfectly balanced, whereas dynamic equilibrium embodies a continuous dance of molecular interactions and energy exchange. In summary, static equilibrium involves no movement or change, while dynamic equilibrium involves movement or change but with no net effect on the overall state of the system. Static equilibrium occurs when all forces on an object are balanced, resulting in no motion relative to a reference frame, while dynamic equilibrium involves balanced forces on a moving object with a constant velocity.
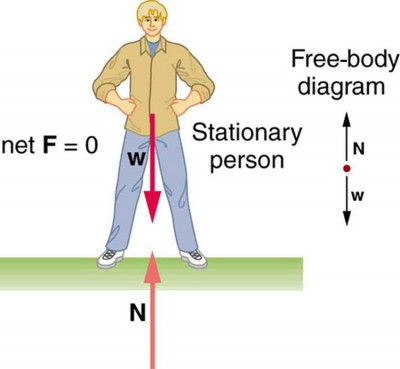
Difference Between Static And Dynamic Equilibrium Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction. static equilibrium, also known as mechanical equilibrium, means the reaction has stopped. in other words, the system is at rest. Static equilibrium represents a state of rest where forces are perfectly balanced, whereas dynamic equilibrium embodies a continuous dance of molecular interactions and energy exchange. In summary, static equilibrium involves no movement or change, while dynamic equilibrium involves movement or change but with no net effect on the overall state of the system. Static equilibrium occurs when all forces on an object are balanced, resulting in no motion relative to a reference frame, while dynamic equilibrium involves balanced forces on a moving object with a constant velocity.
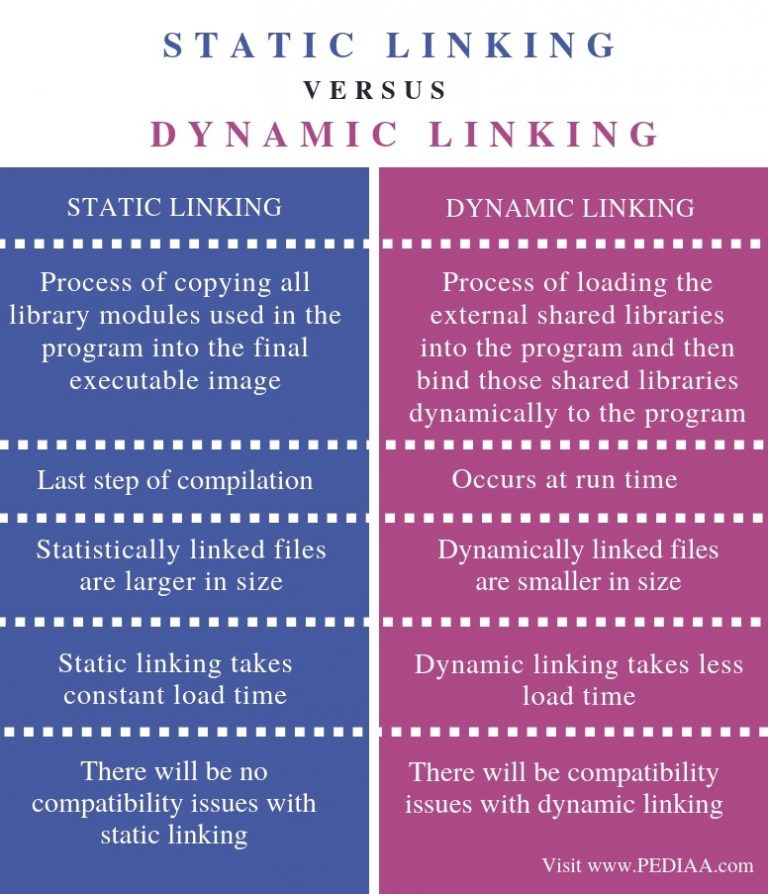
What Is The Difference Between Static And Dynamic Linking Pediaa Com In summary, static equilibrium involves no movement or change, while dynamic equilibrium involves movement or change but with no net effect on the overall state of the system. Static equilibrium occurs when all forces on an object are balanced, resulting in no motion relative to a reference frame, while dynamic equilibrium involves balanced forces on a moving object with a constant velocity.

What Is The Difference Between Static And Dynamic Website Pediaa Com
Comments are closed.